ML385 : NRF2 (Nuclear Factor Erythroid 2-related Factor 2) Inhibitor
ML385
Target Name
Nuclear Factor Erythroid 2-related Factor 2
Target Alias
NRF2
Target Class
Transcription Factor
Mechanism of Action
Inhibitor of NRF2
Biological / Disease Relevance
Non-small cell lung cancer (NSCLC); KEAP1; NRF2
In vitro activity
NRF2-MAFG complex polarization assayIn vitro activity
NRF2-ML385 pull-down assayIn vitro activity
NRF2-ML385 Ni+ affinityIn vitro activity
NRF2-mediated transcription assayIn vivo activity
Pharmacokinetics (T 1/2)In vivo activity
Tumor xenograph assayTarget Information
Loss of function mutations in Kelch-like ECH Associated Protein 1 (KEAP1), or gain-of-function mutations in nuclear factor erythroid 2-related factor 2 (NRF2), are common in non-small cell lung cancer (NSCLC) and associated with therapeutic resistance. To discover novel NRF2 inhibitors for targeted therapy, we conducted a quantitative high-throughput screen using a diverse set of ∼400 000 small molecules (Molecular Libraries Small Molecule Repository Library, MLSMR) at the National Center for Advancing Translational Sciences. We identified ML385 (SID 164194505, CID 1383822) as a probe molecule that binds to NRF2 and inhibits its downstream target gene expression.
Properties
ML385
NCGC00343526
| Physical & chemical properties | ||||
|---|---|---|---|---|
| Molecular Weight | 511.6 g/mol | |||
| Molecular Formula | C29H25N3O4S | |||
| cLogP | 5.3 | |||
| PSA | 109 Ų | |||
| Storage | ||||
| Solubility | ||||
| CAS Number | 846557-71-9 | |||
SMILES:
CC1=CC=CC=C1C(N2CCC3=C2C=CC(C4=C(C)SC(NC(CC5=CC6=C(OCO6)C=C5)=O)=N4)=C3)=O
InChI:
InChI=1S/C29H25N3O4S/c1-17-5-3-4-6-22(17)28(34)32-12-11-20-15-21(8-9-23(20)32)27-18(2)37-29(31-27)30-26(33)14-19-7-10-24-25(13-19)36-16-35-24/h3-10,13,15H,11-12,14,16H2,1-2H3,(H,30,31,33)
InChIKey:
LINHYWKZVCNAMQ-UHFFFAOYSA-N
Activity
Summary activity statement /
ML385 binds to Neh1, the Cap ‘N’ Collar Basic Leucine Zipper (CNC-bZIP) domain of NRF2, and interferes with the binding of the V-Maf Avian Musculoaponeurotic Fibrosarcoma Oncogene Homologue G (MAFG)-NRF2 protein complex to regulatory DNA binding sequences. In clonogenic assays, when used in combination with platinum-based drugs, doxorubicin or taxol, ML385 substantially enhances cytotoxicity in NSCLC cells, as compared to single agents. ML385 shows specificity and selectivity for NSCLC cells with KEAP1 mutation, leading to gain of NRF2 function. In preclinical models of NSCLC with gain of NRF2 function, ML385 in combination with carboplatin showed significant antitumor activity. We demonstrate the discovery and validation of ML385 as a novel and specific NRF2 inhibitor and conclude that targeting NRF2 may represent a promising strategy for the treatment of advanced NSCLC.
In vitro activity - NRF2-MAFG protein complex fluorescence polarization assay
Summary /
ML385 inhibits the ARE NRF2-luc assay in a dose response manner (Figure 1) and directly interacts with NRF2 protein. The addition of ML385 decreased anisotropy in a dose-dependent manner, with an IC50 of 1.9 μM, suggesting that the NRF2-MAFG protein complex was dissociated from fluorescein-labeled ARE-DNA. Importantly, the inactive analog increased anisotropy and did not inhibit the binding of the NRF2-MAFG complex to ARE-DNA duplex (Figure 2a). To demonstrate the direct interaction between ML385 and NRF2 protein, we synthesized biotin-labeled analogs of ML385, including an active biotin analog (AB-ML385) with conjugation through the meta-position of phenyl acetamide and an inactive biotin (IB-ML385) analog. AB-ML385 retained NRF2 inhibitory activity, although with reduced potency, as measured by real time RT-PCR, while IB-385 was inactive (Figure 2b).

Figure 1. Concentration-response curves of ML385 in three ARE NRF2-luc assays (A549, H1437, and H838 cells), HEK293-CMV counter assay and glucocorticoid receptor (GR)-responsive beta-lactamase reporter cell-based assay.

Figure 2. (a) ML385 inhibits DNA binding activity of NRF2-MAFG complex to fluorescein-labeled ARE DNA. Fluorescence intensity was measured to get anisotropy value and IC50 was calculated by fitting of sigmoid curve (R2>0.97). (b) AB-ML385 (10 μM) attenuates NRF2 and GCLc mRNA expression, while IB-ML385 does not. ML385 was used at a concentration of 5 μM. Error bars represent ±S.D. ‘*’P<0.05 relative to vehicle.
In vitro activity - NRF2-ML385 pull-down assay
Summary /
The interaction between AB-ML385 and NRF2 is validated using histidine-tagged human NRF2 with a nickel (Ni+) pull-down assay, followed by the measurement of streptavidin-HRP activity. AB-ML385 showed interaction with NRF2 protein, while IB-ML385 (inactive analog) showed no interaction with NRF2 protein (Figure 3). Importantly, competition with ML385 reduced the interaction between NRF2 protein and AB-ML385 giving a reduced HRP signal. Again, competition with inactive analog had no effect on the NRF2 protein and AB-ML385 interaction suggesting that the interaction between NRF2 and ML385 is specific (Figure 3a). Moreover, the Ni+ affinity pull down assay revealed Neh1, the Cap-n-collar, b-Zip domain of NRF2, as the key domain interacting with AB-ML385 (Figure 3b). The Ni+ affinity pull-down assay using NRF2 protein lacking Neh1 domain (ΔNeh1) showed no interaction with AB-ML385. Taken together, these results suggest that ML385 binds to the Neh1 DNA binding domain of NRF2, blocks the binding of the NRF2-MAFG complex to the ARE sequence in the promoter and reduces transcriptional activity.

Figure 3. ML385 directly interacts with purified NRF2 protein. (a) AB-ML385 binds to purified histidine-tagged full length NRF2 protein in Ni+ pull-down assay and is competed away by ML385. (b) AB-ML385 selectively binds to Neh1 domain of NRF2. NRF2 full-length NRF2 protein; Neh1, Neh1 domain of NRF2, ΔNeh1, full-length NRF2 protein lacking Neh1 domain.
In vitro cellular activity - NRF2-mediated transcription assay
Summary /
ML385 inhibits NRF2 signaling in lung cancer cells with KEAP1 mutations. ML385 showed a dose-dependent reduction in the NRF2 transcription activity and the maximum inhibitory concentration was 5 uM (Figure 4a). A time-dependent decrease in NRF2 signaling and the maximum decline was at 72 h (Figure 4b). In addition to reducing mRNA levels of NRF2 target genes, we observed a reduction in NRF2 mRNA levels (Figure 4d and 4e). These results are in agreement with previous results, which showed that NRF2 auto-regulates its own transcription. Treatment with ML385 also led to diminished NRF2 promoter activity. ML385 treatment significantly attenuated NQO1 enzyme activity and reduced GSH levels along with cellular antioxidant capacity (Figure 4f – 4h).
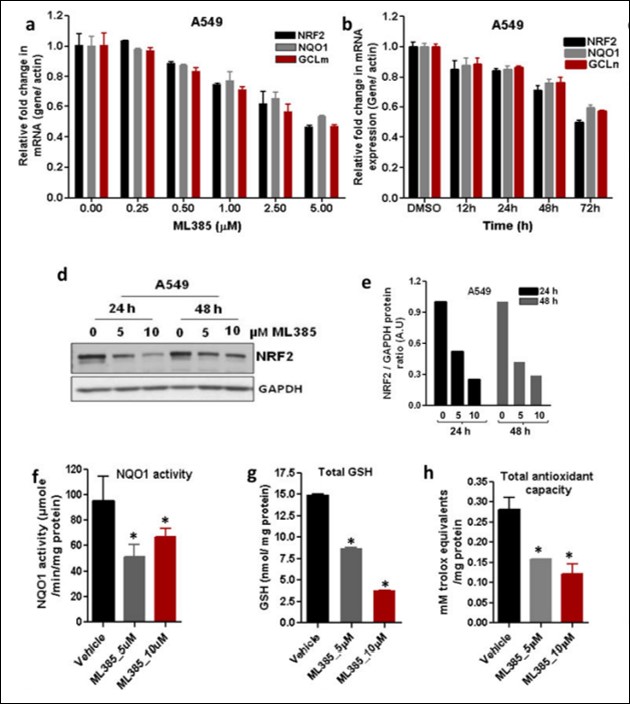
Figure 4. ML385 inhibits NRF2 signaling in lung cancer cells. (a) Dose-dependent inhibition of NRF2-mediated transcription by ML385. Error bars represent ±S.D. (b) Time-dependent reduction in NRF2 and its target genes in A549 cells after treatment with ML385 (5μM). Error bars represent ±S.D. ‘*’P<0.05 relative to vehicle. (d) Immunoblot showing relative levels of NRF2 protein at 24 h and 48 h post-treatment with ML385. (e) Densitometric quantification of NRF2 immunoblot data. (f–h) Treatment with ML385 attenuates antioxidant enzyme activity resulting in lower NQO1 enzyme activity, total antioxidant capacity, and reduced glutathione levels in A549 cells. Error bars represent ±S.D. ‘*’P<0.05 relative to vehicle.
In vitro cellular activity - NSCLC cells (KEAP1 mutant) selective cytotoxicity assay
Summary /
ML385 shows anti-tumor activity in NSCLC both as a single agent and in combination with carboplatin. To determine whether the combination of ML385 and carboplatin observed in cell culture could be recapitulated in vivo, we performed subcutaneous xenograft experiments using A549 and H460 cells. Mice were dosed with ML385, carboplatin, or ML385 in combination with carboplatin for 3–4 weeks and the tumor volumes were measured biweekly. A549 and H460 tumors treated with ML385 in combination with carboplatin showed a significant reduction in tumor growth in both cell lines compared to vehicle. Although the treatment with a single agent (either ML385 or carboplatin) led to a reduction in tumor growth, the magnitude of these effects was variable between cell lines and did not reach statistical significance (Figure 5). These results are consistent with prior findings that were obtained with NRF2 siRNA18 in combination with chemotherapeutic drugs. Tumor samples were analyzed for exposure to ML385 4–6 h post last treatment with ML385. We detected ML385 at intra-tumoral concentrations of ~1 μM in both single agent and combination treatment cohorts. Mice tolerated the combination treatment and the analysis of serum samples for liver and toxicity-related markers revealed no evident signs of toxicity. ML385 in combination with carboplatin led to a significant reduction in tumor cell proliferation, demonstrated by fewer Ki-67 positive cells.

Figure 5. ML385 is selectively toxic to cells with KEAP1 mutations and potentiates the toxicity of chemotherapy drugs in NSCLC cells with KEAP1 mutations. (a) H460, a NSCLC line with a point mutation in KEAP1, is more sensitive to ML385 than H460-KEAP1 Knock-in H460 cells expressing WT KEAP1. ‘*’P<0.05 relative to H460- KEAP1 Knock-in cells. (b) Treatment with ML385 selectively inhibits the colony forming ability of lung cancer cells but has no effect on the growth of non-tumorigenic BEAS2B cells. (c) H460 cells treated with ML385 in combination with chemotherapy drug showed increased caspase 3/7 activity, a marker of apoptosis. Cells treated with chemotherapy drug alone or ML385 in combination with chemotherapy drug were incubated with luminogenic caspase substrate and change in luminescence was measured. Caspase activity was normalized to the number of viable cells using Cell-Titer Blue assay. Error bars represent ±S.D. ‘*’P<0.05 relative to vehicle or ML385; ‘**’ P<0.05 relative to chemotherapy drug alone.
In vivo activity - CD-1 mice pharmacokinetic assay
Summary /
To determine whether ML385 has an appropriate pharmacokinetic (PK) profile for in vivo studies, we dosed CD-1 mice at 30 mg/kg IP. The PK profile showed that ML385 has a half-life (t1/2 = 2.82 h) after IP injection supporting its use in in vivo efficacy studies (Figure 6).

Figure 6. In vivo Pharmacokinetic Profile of ML385 in CD-1 mice. (a) Mice were treated with a single dose of ML385 (30mg/ kg) by Intraperitoneal injection. (b) ML385 Pharmacokinetic simulations at a dose of 30mg/kg to predict tissue concentration of ML385 as a function of time.
In vivo activity - Tumor xenografts and treatment
Summary /
ML385 and carboplatin combination therapy blocks orthotopic human lung tumor growth (Figure 7). Tumor samples treated with ML385 showed a significant reduction in NRF2 protein level and its downstream target genes. Determination of platinum levels in A549 and H460 tumors treated with carboplatin alone or ML385 in combination with carboplatin by inductively-coupled plasma mass spectrometry (ICP-MS) revealed ~2-fold higher platinum levels in tumors treated with combination therapy. Collectively, these results suggest that ML385 potentiates the cytotoxic activity of carboplatin partly by blocking the NRF2-dependent drug detoxification pathway leading to increased drug retention in the tumor. The anti-tumor activity of ML385 in combination with carboplatin was replicated in an independent investigator’s laboratory using H460 xenografts. ML385 in combination with carboplatin has a substantial in vivo efficacy in orthotopic NSCLC models.
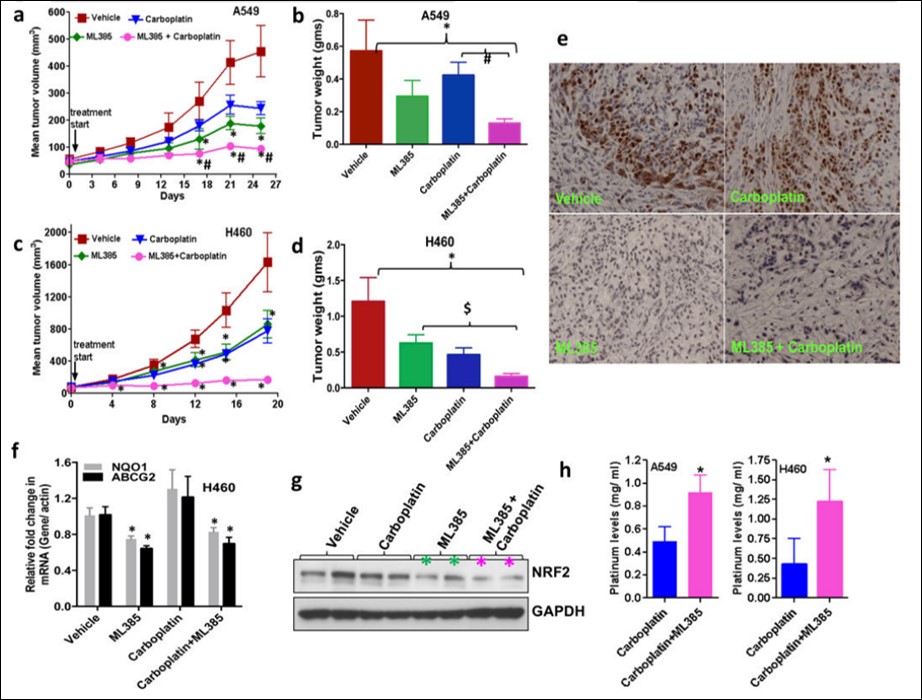
Figure 7. Therapeutic efficacy of ML385 as a single agent and in combination with carboplatin in subcutaneous lung tumor xenografts (a) ML385 shows anti-tumor activity as a single agent in sensitized A549 xenograft tumors to carboplatin therapy. Values represent tumor volume ± S.E.M. for all groups. n = 7 mice/group; ‘*’P<0.05 relative to vehicle; ‘#’ P<0.05 relative to carboplatin. (b) Treatment with ML385 or ML385 in combination with carboplatin significantly reduced tumor weight as compared to vehicle group. Efficacy of ML385 alone was comparable to carboplatin. ‘*’P<0.05 relative to vehicle; ‘#’ P<0.05 relative to carboplatin. (c-d) ML385 sensitized H460 lung tumors to carboplatin treatment. Groups of H460 tumors treated with ML385 or ML385 in combination with carboplatin showed a significant reduction in tumor volume and weight as compared to the vehicle group. Efficacy of ML385 alone was comparable to carboplatin. n = 5–7 mice/group; ‘*’P<0.05 relative to vehicle; ‘$’ P<0.05 relative to ML385. (e) The proliferative index based on Ki-67 immuno-reactivity in A549 subcutaneous tumors. (f) Treatment with ML385 attenuated the expression of NRF2-dependent genes in H460 tumors. ‘*’P<0.05 relative to vehicle or carboplatin (g) Immunoblot showing reduction in NRF2 protein in H460 tumors treated with ML385. (h) Bar graph showing platinum levels in A549 and H460 tumors treated with carboplatin alone or ML385 in combination with carboplatin. ‘*’P<0.05, relative to carboplatin alone.
References
- Nrf2 qHTS screen for inhibitors: Summary
- Singh A, Venkannagari S, Oh KH, Zhang YQ, Rohde JM, Liu L, Nimmagadda S, Sudini K, Brimacombe KR, Gajghate S, Ma J, Wang A, Xu X, Shahane SA, Xia M, Woo J, Mensah GA, Wang Z, Ferrer M, Gabrielson E, Li Z, Rastinejad F, Shen M, Boxer MB, Biswal S. Small Molecule Inhibitor of NRF2 Selectively Intervenes Therapeutic Resistance in KEAP1-Deficient NSCLC Tumors. ACS Chem Biol. 2016 Nov 18;11(11):3214-3225. doi: 10.1021/acschembio.6b00651. Epub 2016 Oct 17. PMID: 27552339; PMCID: PMC5367156.
- Biswal, Shyam et al. NRF2 Small Molecule Inhibitors for Cancer Therapy. WIPO / PCT; Patent WO 2014/145642A9


