ML026 : AmpC (E. Coli AmpC β-Lactamase) Inhibitor
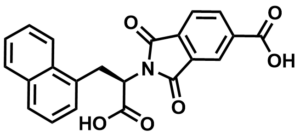
ML026
Target Name
E. Coli AmpC β-Lactamase
Target Alias
AmpC
Target Class
Hydrolase
Mechanism of Action
Inhibitor of AmpC
Biological / Disease Relevance
AmpC beta-lactamase pathway
In Vitro Activity
IC50In Vitro Activity
KiTarget Information
AmpC β-lactamases mostly are cephalosporinases but are capable of hydrolysing all β-lactams to some extent. The hydrolytic properties of AmpC ß-lactamases are similar. Unfortunately, these enzymes have demonstrated drug resistant properties for the third generation cephalosporins presenting immense future challenges to health providers. As such, we explored a screen and optimization program for novel inhibitors (both covalent and non-covalent) of this enzyme class. Non-Covalent inhibitor refers to chemotype 1 of this enzyme class.
Properties

ML026
NCGC00161790
| Physical & chemical properties | ||||
|---|---|---|---|---|
| Molecular Weight | 389.3g/mol | |||
| Molecular Formula | C22H15NO6 | |||
| cLogP | 3.4 | |||
| PSA | 112 | |||
| Storage | ||||
| Solubility | ||||
| CAS Number | ||||
SMILES:
OC([C@H](N1C(C2=C(C1=O)C=C(C(O)=O)C=C2)=O)CC3=C4C=CC=CC4=CC=C3)=O
InChI:
InChI=1/C16H19Cl2N3O5S/c1-16(2,3)26-14(22)19-7-6-13-20-21-15(25-13)27(23,24)9-10-4-5-11(17)8-12(10)18/h4-5,8H,6-7,9H2,1-3H3,(H,19,22)/f/h19H
InChIKey:
KTPSEIKHZBDCBJ-UHFFFAOYSA-N
Activity
Summary activity statement /
ML026 (CID 16752640, SID 26740854) is observed to be an inhibitor of AmpC beta-lactamase. In collaboration with Professor Brian Shoichet, this agent was among the 1274 actives that were characterized by secondary assays, mass analysis, and co-crystallization studies. From this work, several representative phthalimides were noted to be true inhibitors of AmpC including the phenol. A representative structure (SID: 4244870 from initial qHTS) was co-crystallized with Amp C showing a detailed layout of the binding mechanism for this compound class. Additionally, there was no associated mass change for this series of compounds when incubated with AmpC for extended periods of time suggesting that it was inhibiting the enzyme through a non-covalent mechanism (Babaoglu, 2008).
In vitro assay - Selectivity
| ML026 IC50 (uM) | |
|---|---|
|
AmpC beta-lactamase |
5 |
|
Cruzain |
Inactive |
|
Chymotrypsin |
Inactive |
Summary /
ML026 is screened against 2 other targets to evaluate selectivity. ML026 is found to be inactive against Cruzain and Chymotrypsin.
In vitro assay - Co-crystallization studies
Summary /
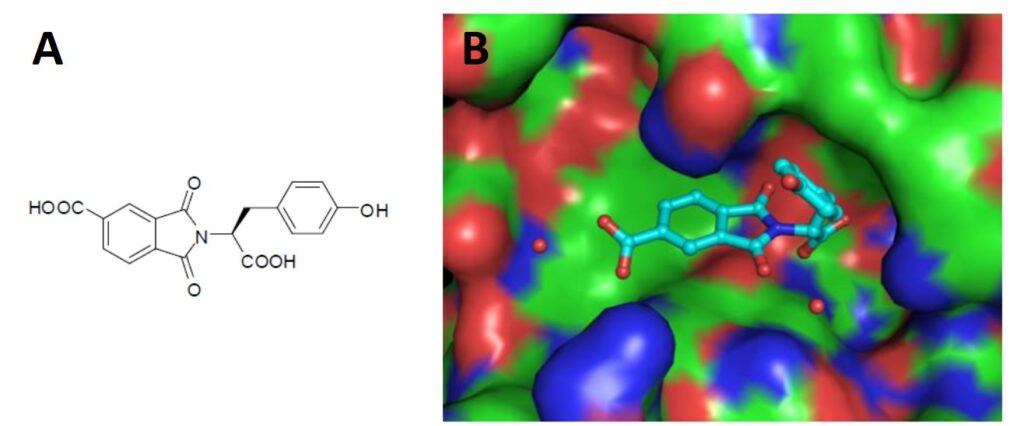
Crystal structure of the lead compound phthalimide (A) into AmpC beta-lactamase (B). Utilizing the crystallographic data, a modest optimization of this structural series was accomplished after making only 20 analogues culminating in SID: 26740854. The stereochemistry of the molecule was noted to be of importance along with the presence of both acid moieties. To this end, we chose to explore only chirally pure analogues of the series as well as a modest phenyl ring scan. These explorations resulted in an optimization of potency for SID: 26740854. PDB 2R9W shows the X-ray structure of the probe bound to the target.


