ML094 : ALOX15 (Arachidonate 15-Lipoxygenase) Inhibitor
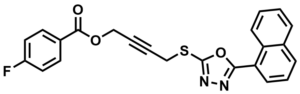
ML094
Target Name
Arachidonate 15-Lipoxygenase
Target Alias
ALOX15
Target Class
Oxygenase
Mechanism of Action
Inhibitor of ALOX15
Biological / Disease Relevance
Prostate tumor development
In vitro activity
15hLO-1 qHTS (IC50)In vitro activity
15hLO-1 Cuvette Assay (IC50)Target Information
Lipoxygenases are a class of non-heme iron-containing enzymes. There are three major human lipoxygenses: 5-, 12, and 15-hLO, whose primary enzymatic difference lies in their site-specific oxidation of arachidonic acid. The products of lipoxygenases are precursors of hormones such as leukotrienes and lipoxins, which have been implicated as critical in a variety of inflammatory diseases and cancers. 15hLO-1 has higher expression in human prostate tumors, compared with adjacent tissue, and this expression correlates with the virulency of the cancer. Also, the 15hLO-1 metabolite of linoleic acid, 13-(S)-hydroxyoctadecadienoic acid, is detected in adenocarcinoma tissue, indicating a pro-tumorigenic role in prostate tumor development for 15hLO-1.
Inhibitors of lipoxygenase are found in three broad categories reflecting the mode of action: reductive, catalytic and allosteric. The reductive inhibitors convert the active, ferric enzyme to the inactive, ferrous form. The catalytic inhibitors act as competitive inhibitors. The allosteric inhibitors are, however, different in their kinetic properties and do not bind to the catalytic site, but an allosteric site, whose exact position is not known. The presence of such a site allows for targeting two sites in lipoxygenase, the allosteric and the catalytic sites, which may have different SARs and different pharmacophore profiles.
Properties

ML094
NCGC00183397
| Physical & chemical properties | ||||
|---|---|---|---|---|
| Molecular Weight | 418.4 g/mol | |||
| Molecular Formula | C23H15FN2O3S | |||
| cLogP | 5.2 | |||
| PSA | 65.22 | |||
| Storage | ||||
| Solubility | 10 mM in DMSO | |||
| CAS Number | ||||
SMILES:
FC1=CC=C(C(OCC#CCSC2=NN=C(C3=C4C=CC=CC4=CC=C3)O2)=O)C=C1
InChI:
InChI=1S/C23H15FN2O3S/c24-18-12-10-17(11-13-18)22(27)28-14-3-4-15-30-23-26-25-21(29-23)20-9-5-7-16-6-1-2-8-19(16)20/h1-2,5-13H,14-15H2
InChIKey:
BZGQMGJZXODGJM-UHFFFAOYSA-N
Activity
Summary activity statement /
ML094 (CID 1998940; SID 99206572) exhibits a potent inhibition of 15hLO-1, yet does not inhibit the closely related 15hLO-2, 12hLO, and 5hLO. This probe is the most 15hLO-1-selective inhibitor reported to date. Additionally, this probe and its analogs were found to be non-reductive and exhibited reversible inhibition, with inhibitor binding occurring at both the catalytic site and possibly the allosteric site (Rai 2010). ML094 can then be useful for in vitro studies of 15hLO-1, but may also be utilized as a starting point for the development of 15hLO-1 effectors in cellular or animal models.
In vitro activity - Selectivity Assay
| ML094 | BMS Compound (Control) | |
|---|---|---|
|
15hLO-1 (IC50) |
14 nM | 21 nM |
|
5hLO (IC50) |
> 50000 nM | > 3000 nM |
|
12hLO (IC50) |
>100000 nM | > 3000 nM |
|
15hLO-2 (IC50) |
> 500000 nM | NA |
|
COX-1 (IC50) |
inactive at 10 uM | NA |
|
COX-2 (IC50) |
inactive at 10 uM | NA |
Summary /
ML094 exhibit >7500-fold selectivity over similar hLO isozymes (5, 12, and 15 hLO-2) and COX isozymes (inhibition less than 5% of total activity). Moreover, potency towards 15hLO-1 and selectivity vs. other isozymes are better than prior art Bristol-Myers Squibb (BMS) compound (Weinstein 2005).
References
- Probe Development Summary of Inhibitors of 15-hLO-1 (15-human lipoxygenase 1)
- Rai G, Kenyon V, Jadhav A, Schultz L, Armstrong M, Jameson JB, Hoobler E, Leister W, Simeonov S, Holman TR, and Maloney DJ. Discovery of Potent and Selective Inhibitors of Human reticulocyte 15-Lipoxygenase-1. Journal of Medicinal Chemistry 2010 53 (20), 7392-7404
- Rai G, Kenyon V, Jadhav A, Schultz L, Armstrong M, Jameson JB, Hoobler E, Leister W, Simeonov S, Holman TR, and Maloney DJ. Supporting Information of Discovery of Potent and Selective Inhibitors of Human reticulocyte 15-Lipoxygenase-1. Journal of Medicinal Chemistry 2010 53 (20), 7392-7404
- Weinstein DS, Liu W, Gu Z, et al. Tryptamine and homotryptamine-based sulfonamides as potent and selective inhibitors of 15-lipoxygenase. Bioorg Med Chem Lett. 2005;15(5):1435-1440. doi:10.1016/j.bmcl.2004.12.081
- Vasquez-Martinez Y, Ohri RV, Kenyon V, Holman TR, Sepúlveda-Boza S. Structure-activity relationship studies of flavonoids as potent inhibitors of human platelet 12-hLO, reticulocyte 15-hLO-1, and prostate epithelial 15-hLO-2. Bioorg Med Chem. 2007 Dec 1;15(23):7408- 25


