ML084 : COPA (Golgi Type I coat) Modulator
ML084
Target Name
Golgi Type I coat
Target Alias
COPA
Target Class
Vesicle Coat Protein
Mechanism of Action
Modulator of COPA
Biological / Disease Relevance
Lipid storage, Protein trafficking pathway
In vitro activity
COPI AC50Target Information
Storing lipids as a reservoir for energy or the anabolism of elementary metabolites is a common feature of life in organisms from bacteria to humans. The universal cellular lipid storage organelle is the so-called lipid storage droplet (LD). Despite their ubiquitous nature, LDs share a simple, stereotyped structure of a hydrophobic core harboring the storage lipids, which is shielded by a droplet-specific phospholipid monolayer to which proteins are attached. The current model of LD biogenesis involves an incorporation of the lipid core into the membrane leaflets of the endoplasmic reticulum (ER), followed by a subsequent budding-like maturation of a LD, which ultimately pinches off. Once released, LD volume can increase by localized lipogenesis or fusion of existing droplets. Storage lipids are re-mobilized enzymatically by lipase activity. Lipase regulation in the adipocyte is heavily studied and involves multiple components including catecholamine signaling (Langin 2006), the LD-associated proteins Perilipin and comparative gene identification 58 (CGI-58) (Subramanian 2004), and at least two lipases named hormone sensitive lipase (HSL) (Vaughan, 1964) and adipocyte triglyceride lipase (ATGL) (Gronke 2005, Haemmerle 2006, Zimmermann 2004). LD biogenesis, turnover and mobilization are poorly understood and only few components are known. However, there is an urgent need to learn more about ectopic fat depots as mislocalized storage of lipids, for example in the liver or muscle, is an eminent health problem associated with insulin resistance or the metabolic syndrome (Frayn 2006). The chemical probes yielded by this project should be useful tools for providing a better understanding of cellular and organismic lipid storage on a functional and evolutionary level. Furthermore, active substances might result in the identification of lead compounds for the treatment of emerging lipid storage-associated diseases, including atherosclerosis, diabetes or obesity.
ML084 came from initial screening of known bioactive compound collections. By combining the small molecule screening results with lipid metabolism modulating gene functions identified in the genome-wide RNAi screen, we were able to identify COPI proteins as negative regulators of lipid storage and the probe ML084 (Exo1) as chemical probe to modulate lipid storage.
Project Team
Properties

ML084
Exo1
| Physical & chemical properties | ||||
|---|---|---|---|---|
| Molecular Weight | 273.26 g/mol | |||
| Molecular Formula | C15H12FNO3 | |||
| cLogP | 3.4 | |||
| PSA | 55.4 | |||
| Storage | Store at room temperature | |||
| Solubility | Max concentration: 100 mM in DMSO; 25 mM in ethanol with gentle warming | |||
| CAS Number | ||||
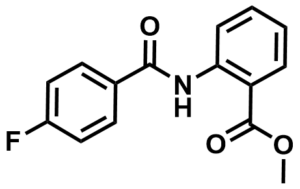
SMILES:
COC(C1=C(NC(C2=CC=C(F)C=C2)=O)C=CC=C1)=O
InChI:
InChI=1S/C15H12FNO3/c1-20-15(19)12-4-2-3-5-13(12)17-14(18)10-6-8-11(16)9-7-10/h2-9H,1H3,(H,17,18)
InChIKey:
KIAPWMKFHIKQOZ-UHFFFAOYSA-N
Activity
Summary activity statement /
The probe Exo-1 is a modulator of protein trafficking. Our work has linked the Golgi Type I coat proteins known as cotamer, COPI to lipid storage. Exo-1 can be used to induce a lipid storage phenotype in cells through modulation of the protein trafficking pathways.
In vitro activity - Confirmatory Assay
| ML084 (AC50) | |
|---|---|
|
COPI |
5 +/- 2.5 uM |
|
S3 cells Cytotoxicity |
Inactive at 40 uM |
Summary /
Compounds showing decreasing lipid storage were generally associated with cytotoxicity, except for the positive control Triacsin C. ML084, is found to modulate lipid storage without cytotoxic effect.
In vitro activity - Modes of Action
Summary /
ML084 (Exo1) is also observed to be an Arf1 modulator (Feng 2003) that increased lipid droplet accumulation (EC50 = 5 μM) in S3 cells. This compound is known to inhibit the vesicle-mediated Coat Protein complex I (COPI) transport complex. Further, we have identified key Drosophila candidate genes for lipid droplet regulation by RNA interference (RNAi) screening that included the COPI transport complex, which was found to be required for limiting lipid storage. We found that interference with COPI function by RNAi in Drosophila Kc167 cells, as well as in mouse 3T3-L1 or AML-12 cells, results in increased lipid storage (Beller 2008).
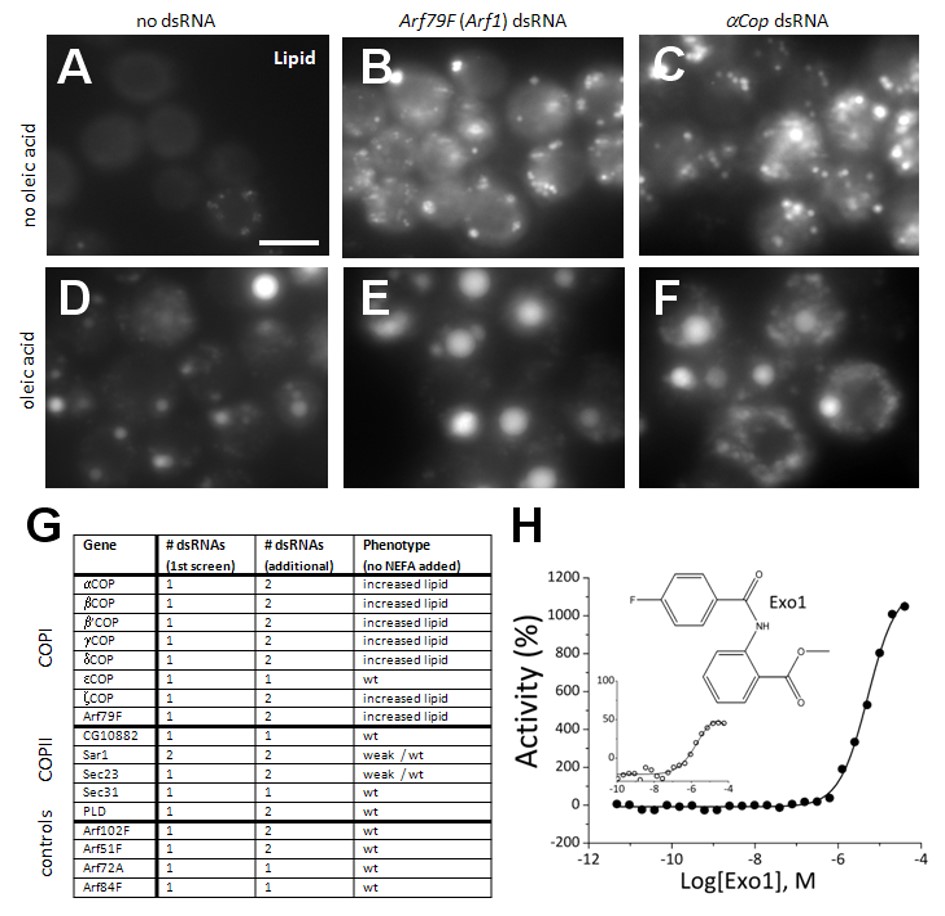
Figure 1. The COPI-Mediated Retrograde Trafficking Pathway is a negative regulator of lipid storage (A–F) Drosophila cells with or without oleic acid stained with BODIPY493/503 to detect lipid. Control cells not treated with dsRNA (A and D) or cells incubated with dsRNAs targeting Arf79F (an Arf1 homolog) (B and E) or alphaCop (C and F) are shown. (G) All COPI, and several COPII members as well as additional Arfs, were retested using independent dsRNAs and gave similar results. Results and number of dsRNAs present in the primary screen (including oleic acid) and retests are given. (H) Dose response of Drosophila S3 cells to Exo1 (structure inset) showing the % activity derived either from the lipid specific signal (filled circles) or the lipid/cell ratio (open circles). Percent activity refers hereby to the changes of lipid storage relative to Triacsin C treatment, which decreases lipid storage by blocking TG synthesis. Increased activity indicates increased lipid storage, which increased with concentration. Scale bar in (A) represents 10 (Beller 2008).
In vitro activity - Lipolysis Assay in AML12 cells
Summary /
In order to characterize the COPI knockdown effects on lipid storage in greater detail, and to confirm the independently identified effect of the Exo1 compound, we utilized different secondary assays. In addition to Exo1, we used BFA, another compound that has been implicated in the modulation of COPI-mediated trafficking in mammalian cells (Lippincott-Schwartz 1991). In order to support our finding of evolutionary conservation of COPI effects on lipid storage, we also utilized Exo1 and BFA in the mammalian cell system. Both compounds reduced NEFA release to the same extent as the siRNAs targeting COPI subunit encoding mRNAs. Mimicking genetic epistasis experiments by combining RNAi and compound treatment, we furthermore obtained results supporting the hypothesis that COPI functions in the same pathway as ATGL and might be involved in its activation, opening up new possibilities to study this currently heavily studied question (Figure 2). Positive regulation of lipolysis by the COPI retrograde vesicle trafficking pathway was the most striking and unexpected result of both the RNAi and small molecule compound screen. We propose that COPI is likely to function directly at the lipid droplet surface and rather than indirectly through the Golgi (Beller 2008).
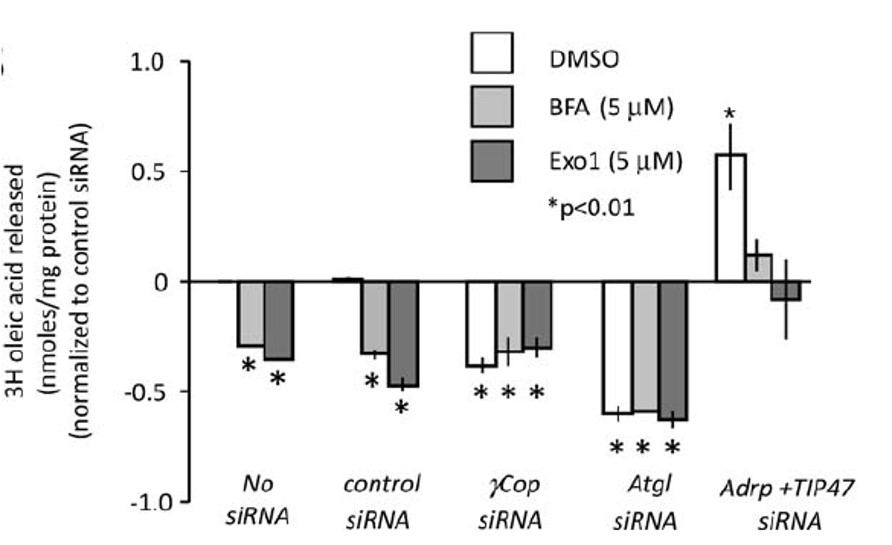
Figure 2. NEFA Incorporation and NEFA Release Measured in AML12 Cells after compound treatment. Relative activity [(experimental/ALLStars negative control (control) and DMSO) Radiolabel assays for NEFA release (nM) relative to total protein concentration in cells treated with siRNAs targeting the indicated transcripts in the presence of DMSO only (open bar), BFA (5 μM) in DMSO (light-grey bar), or Exo1 (5 μM) in DMSO (dark-grey bar). Significance at p<0.01, unpaired t-test, is shown (indicated by an asterisk [*]). Standard error is indicated by the bars.
References
- qHTS Assay for Lipid Storage Modulators: Summary
- Langin, D. Adipose tissue lipolysis as a metabolic pathway to define pharmacological strategies against obesity and the metabolic syndrome. Pharmacol Res 2006, 53, 482-91
- Subramanian, V.; Rothenberg, A.; Gomez, C.; Cohen, A. W.; Garcia, A.; Bhattacharyya, S.; Shapiro, L.; Dolios, G.; Wang, R.; Lisanti, M. P.; Brasaemle, D. L. Perilipin A mediates the reversible binding of CGI-58 to lipid droplets in 3T3-L1 adipocytes. J Biol Chem 2004, 279, 42062-71
- Vaughan, M.; Berger, J. E.; Steinberg, D. Hormone-Sensitive Lipase and Monoglyceride Lipase Activities in Adipose Tissue. J Biol Chem 1964, 239, 401-9
- Gronke, S.; Mildner, A.; Fellert, S.; Tennagels, N.; Petry, S.; Muller, G.; Jackle, H.; Kuhnlein, R. P. Brummer lipase is an evolutionary conserved fat storage regulator in Drosophila. Cell Metab 2005, 1, 323-30
- Haemmerle, G.; Lass, A.; Zimmermann, R.; Gorkiewicz, G.; Meyer, C.; Rozman, J.; Heldmaier, G.; Maier, R.; Theussl, C.; Eder, S.; Kratky, D.; Wagner, E. F.; Klingenspor, M.; Hoefler, G.; Zechner, R. Defective lipolysis and altered energy metabolism in mice lacking adipose triglyceride lipase. Science 2006, 312, 734-7
- Zimmermann, R.; Strauss, J. G.; Haemmerle, G.; Schoiswohl, G.; Birner- Gruenberger, R.; Riederer, M.; Lass, A.; Neuberger, G.; Eisenhaber, F.; Hermetter, A.; Zechner, R. Fat mobilization in adipose tissue is promoted by adipose triglyceride lipase. Science 2004, 306, 1383-6
- Frayn, K. N.; Arner, P.; Yki-Jarvinen, H. Fatty acid metabolism in adipose tissue, muscle and liver in health and disease. Essays Biochem 2006, 42, 89-103
- Feng, Y.; Yu, S.; Lasell, T. K.; Jadhav, A. P.; Macia, E.; Chardin, P.; Melancon, P.; Roth, M.; Mitchison, T.; Kirchhausen, T. Exo1: a new chemical inhibitor of the exocytic pathway. Proc Natl Acad Sci U S A 2003, 100, 6469-74
- Beller, M.; Sztalryd, C.; Southall, N.; Bell, M.; Jackle, H.; Auld, D. S.; Oliver, B. COPI complex is a regulator of lipid homeostasis. PLoS Biol 2008, 6, 2530-2549


