ML102 : EGFR (Epidermal Growth Factor Receptor) Inhibitor

ML102
Target Name
Epidermal Growth Factor Receptor
Target Alias
EGFR
Target Class
Transmembrane Signal Receptor
Mechanism of Action
Inhibitor of EGFR
Biological / Disease Relevance
ERK signaling pathway; Cancer cell proliferation, differentiation, development, and survival
In vitro activity
EGFR qHTS (IC50)In vitro activity
EGFR-T790M (IC50)In vitro activity
EGFR-L858R (IC50)Target Information
The ERK phosphorylation signaling pathway plays a significant role in regulation of cellular functions. The anomalous activation of the ERK pathway in cancer has been shown to promote cell proliferation, differentiation, development and survival (Lee 2002, Thompson 2005). ERK phosphorylation can be activated by upstream receptor tyrosine kinases (RTK), and therefore provides a convenient tool to screen members of these receptor families. Advent of a novel cell-based assay detecting ERK phosphorylation allows for the screening of membrane permeable inhibitors of the ERK signaling pathway. In the present assay, signaling of the ERK pathway is triggered by the activation of the epidermal growth factor receptor (EGFR). Currently, there are three well-known EGFR antagonists in clinical use: Erlotinib, Gefitinib and Lapatinib. Lapatinib is an orally active drug used in the treatment of solid tumors, such as breast cancer. Erlotinib and Gefitinib are being used to treat cancer patients with metastatic non-small cell lung cancer (NSCLC), where EGFR over-expression is the target of inhibition. However, mutations in EGFR of NSCLC-carrying patients have been shown to render the enzyme non respondent to Erlotinib and Gefitinib treatments (Engelman 2008).
ML102 is identified as an inhibitors of the ERK pathway using a novel cell-based ERK phosphorylation assay. This probe is shown to inhibit EGFR; and more significantly, also inhibited clinically observed EGFR mutants (EGFR L858R, EGFR T790M, EGFR L858R T790M) with similar or even greater potencies.
Properties

ML102
MLS000391787
| Physical & chemical properties | ||||
|---|---|---|---|---|
| Molecular Weight | 278.31 g/mol | |||
| Molecular Formula | C16H14N4O | |||
| cLogP | 2.6 | |||
| PSA | 65.8 | |||
| Storage | ||||
| Solubility | ||||
| CAS Number | ||||
SMILES:
CC1NC2=C(NC(/C1=N\NC3=CC=CC=C3)=O)C=CC=C2
InChI:
InChI=1S/C16H14N4O/c1-11-15(20-19-12-7-3-2-4-8-12)16(21)18-14-10-6-5-9-13(14)17-11/h2-10,19H,1H3,(H,18,20,21)
InChIKey:
RWIQZKLIGWLCEK-UHFFFAOYSA-N
Activity
Summary activity statement /
ML102 (SID 22409543; CID 2303746) has been identified in a cell-based ERK phosphorylation assay as an inhibitor for the ERK phosphorylation pathway. The subsequent enzyme assay confirmed its selective inhibitory activity on the EGFR tyrosine kinase. In addition, ML102 inhibits the kinase activities of several EGFR mutants (EGFR L858R, EGFR T790M, EGFR L858R T790M) with the potency equal to or up to 4 times greater than what was observed with the wild type EGFR receptor. ML102 satisfied the project goal of a novel chemotype that is a selective inhibitor of EGFR, is cell membrane permeable, and was the most potent member of this series.
In vitro activity - Selectivity Assay
| ML102 (IC50) | |
|---|---|
|
EGFR qHTS |
0.710 uM |
|
c-Raf |
> 100 uM |
|
Mek-1 |
> 100 uM |
Summary /
ML102 was assayed against purified EGFR tyrosine kinase, Raf and MEK kinases to characterize compound selectivity. The probe molecule is found to exhibit > 100 fold selectivity towards the EGFR than c-Raf or MEK.
In vitro activity- Kinase Profiling Assay
Summary /
ML102 profiling from Ambit Reaction Biology confirmed inhibition of EGFR with nano molar potency. The results compare favorably with the in-house determination of activity. Furthermore, ML102 inhibits several clinically relevant EGFR mutants. By itself, EGFR kinase inhibition is not a novel activity for a chemical series. There are three such compounds now approved for marketing: gefitinib, erlotinib, and lapatinib. A well known limitation of such compounds (Li 2008) is that certain mutations in EGFR prevent the existing compounds from inhibiting the enzyme. ML102 does not suffer from this limitation (Figure 1). Staurosporine also retains potency against these mutants, but its lack of selectivity against other kinases as well as its pharmacodynamic properties has made it a challenging candidate for development. ML102 represents a novel chemotype for kinase inhibition and has a promising profile as an EGFR kinase inhibitor (Table 1).
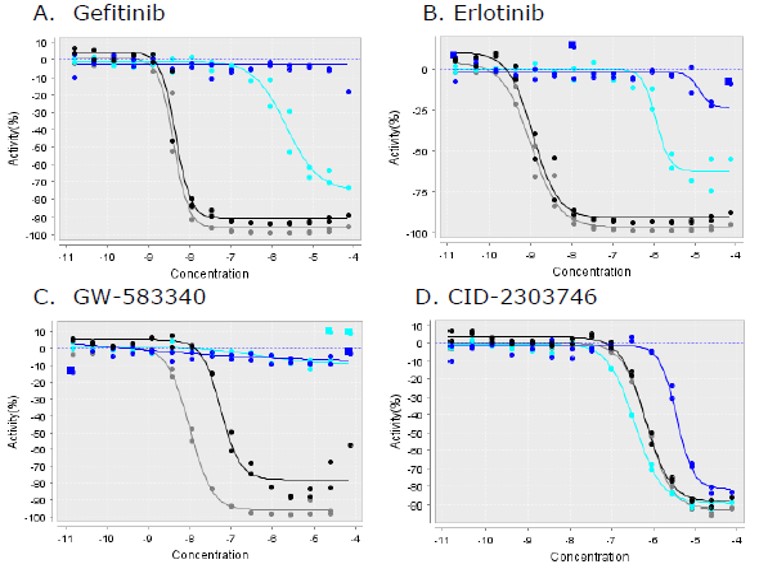
Figure 1. Activity of probe and other EGFR inhibitors against clinical EGFR mutants. GW-583340 is a close analog of lapatinib. Black = L858R, Cyan = T790M, Blue = T790M/L858R, Grey = wild type enzyme.
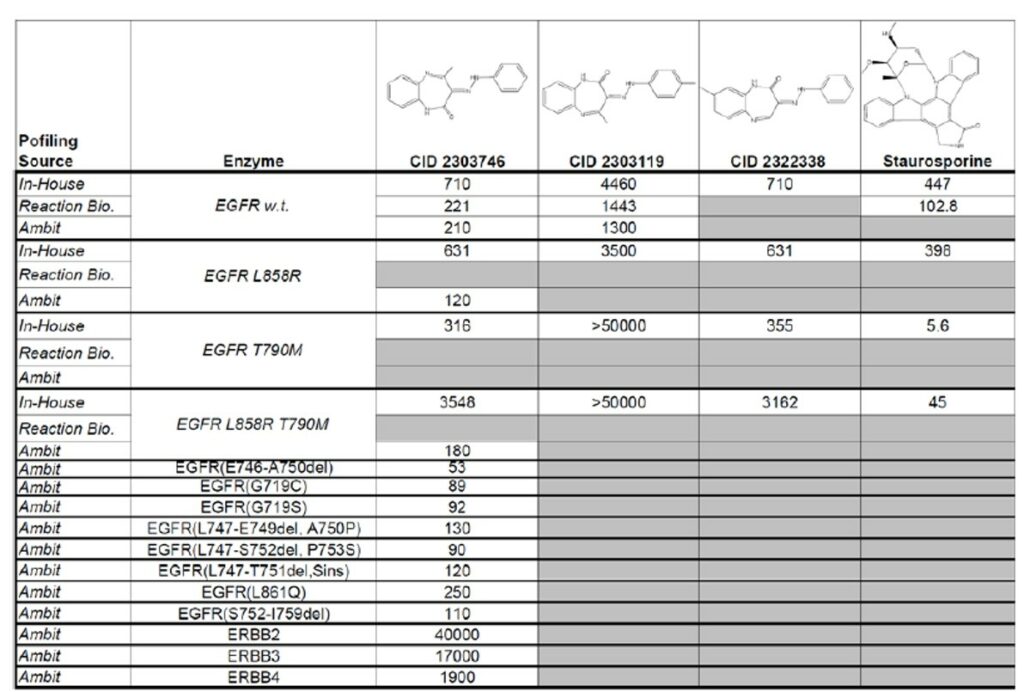
Table 1. Summary of CID-2303746 (ML102) and analogs’ activity against EGFR, clinically observed mutants of EGFR, and homologous enzymes ERBB2, ERBB3, and ERBB4 based on data generated in-house, and at Ambit and Reaction Biology.
References
- Quantitative High-Throughput Screen for Inhibitors of the ERK Signaling Pathway using a Homogeneous Screening Assay: Summary
- Marugan J, Dehdashti S, Zheng W, et al. HTS for Identification of Inhibitors against the ERK Signaling Pathway using a Homogenous Cell-based Assay. 2009 May 18 [Updated 2010 Sep 2]. In: Probe Reports from the NIH Molecular Libraries Program [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2010
- Engelman JA, Jänne PA. Mechanisms of acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in non-small cell lung cancer. Clin. Cancer Res. 2008, 14(10):2895-2899.
- Li D, Ambrogio L, Shimamura T, Kubo S, Takahashi M, Chirieac LR, Padera RF, Shapiro GI, Baum A, Himmelsbach F, Rettig WJ, Meyerson M, Solca F, Greulich H, Wong KK. BIBW2992, an irreversible EGFR/HER2 inhibitor highly effective in preclinical lung cancer models. Oncogene. 2008, 27(34):4702-4711
- Lee JT Jr, McCubrey JA. The Raf/MEK/ERK signal transduction cascade as a target for chemotherapeutic intervention in leukemia. Leukemia. 2002, 16:486-507
- Thompson N, Lyons J. Recent progress in targeting the Raf/MEK/ERK pathway with inhibitors in cancer drug discovery. Curr Opin Pharmacol. 2005, 5:350-356


