ML103 : Tau (Tau) Inhibitor
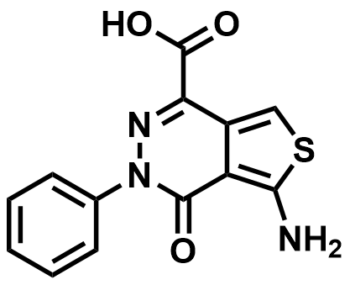
ML103
Target Name
Tau
Target Alias
Tau
Target Class
Microtubule-associated Protein
Mechanism of Action
Inhibitor of Tau
Biological / Disease Relevance
Alzheimer's Disease, Taupathies and tau oligomerization
In vitro activity
Human Tau Oligomerization (IC50)In vitro activity
Tau Sedimentation Assay (% Inhibition)Target Information
Tau becomes pathologically hyperphosphorylated in AD, resulting in reduced MT-binding, followed by aggregation and sequestration of tau as paired helical filaments (PHFs or PHFtau) into neurofibrillary tangles (NFTs). This loss of tau function leads to destabilization of the MT system, which is essential for axonal transport of proteins and other cargo to and from the cell body of neurons. By analogy with a railway, tau functions like the cross ties on railroad tracks (MTs), upon which trains (molecular motors) convey cargo (organelles, proteins) to and from nodes on the railway network (destinations in neuronal perikarya and their processes); the loss of a critical number of cross ties (the consequence of converting tau into PHFs) results in dissolution of the railroad tracks, leading to the failure to deliver cargo to assigned destinations (impaired axonal transport). The deleterious consequences of these events are the dysfunction and subsequent death of affected neurons. Recent advances in the development of in vitro tau fibrillization assays enable HTS to be used for interrogating compound libraries to identify fibrillization inhibitors.
Probe ML103 is member of a series of tau oligomerization/fibrillization inhibitors. It can be used to study tau protein aggregation in vitro, as well as a starting point for drug development for the treatment of Alzheimer’s Disease (AD) and other tauopathies.
Properties

ML103
aminothienopyridazine (ATPZ)
| Physical & chemical properties | ||||
|---|---|---|---|---|
| Molecular Weight | 287.3 g/mol | |||
| Molecular Formula | C13H9N3O3S | |||
| cLogP | 2.3 | |||
| PSA | 124 | |||
| Storage | stable at room temperature | |||
| Solubility | 10 mM in DMSO | |||
| CAS Number | ||||
SMILES:
NC(SC=C1C(C(O)=O)=NN2C3=CC=CC=C3)=C1C2=O
InChI:
InChI=1S/C13H9N3O3S/c14-11-9-8(6-20-11)10(13(18)19)15-16(12(9)17)7-4-2-1-3-5-7/h1-6H,14H2,(H,18,19)
InChIKey:
XOQUOBZQPFFOSV-UHFFFAOYSA-N
Activity
Summary activity statement /
ML103 (SID 57288397; CID 9795907) belongs to the aminothienopyridazines (ATPZ’s) compound series. Its ability to inhibit tau fibril formation was evaluated in the K18PL fluorescence and sedimentation assays to determine their IC50 values and percent maximal inhibition (PubChem AID-1558; AID-1720). Although previous studies have demonstrated that tau fragments such as K18PL form fibrils which resemble those observed in tauopathies (Crowe 2007), it was important to demonstrate that this compound could disrupt assembly of full-length tau40. Two ATPZ compounds (NCGC00053250 and NCGC0031883) were tested at 50 uM concentration and caused at least a 60% inhibition of ThT fluorescence in the tau40 assay. This result confirms that ATPZ’s affect full-length tau in addition to the truncated tau fragment. Since an important function of tau protein is to stabilize microtubules (MTs), we evaluated whether these inhibitors interfered with the ability of tau to promote tubulin polymerization into MTs. Unlike methylene blue, a promiscuous compound active in a high percentage of PubChem screens, the ATPZ’s had no effect on tau-mediated tubulin polymerization indicating that the probe didn’t interfere with the MT assembly (Crowe 2009).
In vitro activity - Selectivity Assay
| ML103 (IC50 / % Inhibition) | |
|---|---|
|
Tau Oligomerization |
6.3 uM / 100% |
|
A-beta (1-42) |
35.5 uM / 24% |
Summary /
ML103 and its ATPZ analogs were also tested for their ability to block the fibrillization of A-beta (1-42). We found that these compounds were less effective in blocking A-beta (1-42) fibril formation,than they were in inhibiting tau fibrillization (Figure 1) with a 5.6 fold selectivity. In addition even at 80 uM treatement, ML102 didn’t cause > 45% inhibition A-beta (1-42) fibril formation.
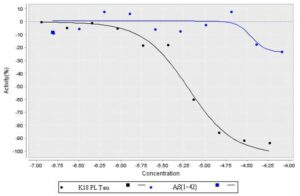
Figure 1. Comparison of K18PL tau and A-beta (1-42) fibrillization in the presence of probe ML102. The probe was tested at the indicated concentrations in ThT binding assays for K18PL tau and A-beta (1-42) fibril formation. Result showed inhibition of the Tau fibrilization (100%) compared to A-beta (<30% at max).
References
- Quantitative High-Throughput Screen for Inhibitors of Tau Fibril Formation: Summary
- Crowe A, Huang W, Ballatore C, et al. Identification of aminothienopyridazine inhibitors of tau assembly by quantitative high-throughput screening. Biochemistry. 2009;48(32):7732-7745. doi:10.1021/bi9006435
- Crowe, A., C. Ballatore, et al. (2007). "High throughput screening for small molecule inhibitors of heparin-induced tau fibril formation." Biochem Biophys Res Commun 358(1): 1-6
- Khlistunova, I., J. Biernat, et al. (2006). "Inducible expression of Tau repeat domain in cell models of tauopathy: aggregation is toxic to cells but can be reversed by inhibitor drugs." J Biol Chem 281(2): 1205-14


