ML132 : CASP1 (Caspase-1) Inhibitor
ML132
Target Name
Caspase-1
Target Alias
CASP1
Target Class
Protease
Mechanism of Action
Inhibitor of CASP1
Biological / Disease Relevance
caspase 1 inhibitor, Cysteine proteases, Caspase 1
In vitro activity
Caspase 1 qHTS (IC50)Target Information
Caspase 1, also known as interleukin-converting enzyme or ICE, is responsible for the proteolytic activation of interleukin (IL)-1-beta and IL-18 (Matinon 2004). IL-1-beta and IL-18 are cytokines that play a major role in the immune response and within numerous autoimmune and inflammatory diseases. Caspase 1 is constitutively and inducibly expressed in immune response elements such as T cells, macrophages and neutrophils. Inhibitors of caspase 1 are sought for intervention strategies within ischemic disorders, Huntington’s disease, amyotrophic lateral sclerosis (ALS), rheumatoid arthritis, osteoarthritis, inflammatory bowel disease and sepsis.
A nitrile-containing propionic acid moiety as an electrophile for covalent attack by the active site cysteine residue of caspase 1 was investigated. Several cyanopropanate containing small molecules were synthesized, including one based upon the optimized peptidic scaffold of the prodrug VX-765. A number of these compounds were potent inhibitors of caspase 1 (IC50s ≤ 1 nM). Examination of these small molecules versus a caspase panel demonstrated an impressive degree of selectivity for caspase 1 inhibition. The small molecular probe ML132 (CID-4462093; NCGC-00183434) is the most potent caspase 1 inhibitor reported to date. It also possesses a unique selectivity pattern relative to other reported caspase inhibitors. A number of these compounds were assessed for their hydrolytic stability and selected ADME properties.
Properties

ML132
NCGC00183434
| Physical & chemical properties | ||||
|---|---|---|---|---|
| Molecular Weight | 477.9 g/mol | |||
| Molecular Formula | C22H28ClN5O5 | |||
| cLogP | 1.6 | |||
| PSA | 166 | |||
| Storage | ||||
| Solubility | 10mM in DMSO | |||
| CAS Number | ||||
SMILES:
CC(C)([C@@H](C(N1CCC[C@H]1C(N[C@H](C#N)CC(O)=O)=O)=O)NC(C2=CC(Cl)=C(N)C=C2)=O)C
InChI:
InChI=1S/C22H28ClN5O5/c1-22(2,3)18(27-19(31)12-6-7-15(25)14(23)9-12)21(33)28-8-4-5-16(28)20(32)26-13(11-24)10-17(29)30/h6-7,9,13,16,18H,4-5,8,10,25H2,1-3H3,(H,26,32)(H,27,31)(H,29,30)/t13-,16-,18+/m0/s1
InChIKey:
KENKPOUHXLJLEY-QANKJYHBSA-N
Activity
Summary activity statement /
ML132 (NCGC 00183434; CID 44620939; SID 87544173) contains nitrile and acid moieties. The nitrile group is intended to be a covalent modifier of the target caspase 1. Often, covalent modifiers are avoided due to promiscuity issues and potential for toxicities within in vivo studies. However, a large number of known drugs do indeed inhibit their target via covalent modification at the orthosteric site, and as tool compounds, covalent modifiers can be very useful if their selectivity can be shown versus related targets.
In vitro activity - Selectivity Assay (Caspase Profiling)
| Reaction Biology Profile | ML132 (IC50) |
|---|---|
|
Caspase 1 |
0.023 nM |
|
Caspase 4 |
14.5 nM |
|
Caspase 8 |
3 nM |
|
Caspase 6 |
10000 nM |
|
Caspase 9 |
5.07 nM |
|
Caspase 5 |
10.6 nM |
|
Caspase 10 |
66.5 nM |
|
Caspase 14 |
801 nM |
Summary /
The selectivity of ML132 is profiled against a commercial panel of caspases offered by Reaction Biology Corporation. The results demonstrated an impressive potency against caspase 1 (IC50 = 0.023nM) with > 1000 fold selectivity. All other activities were above the 1μM threshold.
In vitro cellular activity - ADME Profiling
| ADME Profiling | ML132 |
|---|---|
|
Caco (A->B) Papp (x10^-6 cms^-1) |
0.144 |
|
Caco (B->A) Papp (x10^-6 cms^-1) |
0.060 |
|
Protein Binding (fraction unbound) |
0.431 |
|
Microsomal Stability CLint (uL/min/mg protein) |
10.3 |
|
Microsomal Stability t1/2 (min) |
134 |
Summary /
ML132 was submitted to Cyprotex for a profile of bi-directional Caco-2 permeability, plasma protein binding (both human and rat) and microsomal stability (both human and rat) studies. The probe showed relatively low A to B permeability. The free acids in the probe had a significantly higher free fractions in both human and rat protein binding assays. The clearance rates (Clint) and t1/2 for the probe is moderate.
In vitro activity - Mechanism of Action Studies
Summary /
The binding mechanism of ML132 is studied through molecular modeling using 2HBQ, a co-crystal of caspase 1 and Z-VAD-FMK (Romanowski 2004, Wilson 1994, Okamoto 1999). The presumption of a covalent reversible mechanism of inhibition when building a model for binding the probe is used. The nitrile carbon was therefore held at a proximal distance (2.6 Å) from the catalytic cysteine residue (C285) by constraint docking, and flexibility was granted to the remainder of the small molecule to achieve an optimal binding pose using FRED12. The results demonstrate complementarity between the peptidic fragment of ML132 and the peptide binding domain of caspase 1. Key interactions were noted for the acid moiety and arginine residues 341 and 179 in similar fashion to other Asp containing small molecule caspase 1 inhibitors. While direct interrogation of a covalent interaction between the nitrile and C285 was not pursued in our model, this representation does illustrate the open binding cavity that accommodates the tetrahedral intermediate that forms as a result of covalent binding with aldehyde based inhibitors (a mimetic of the hemithiolacetal intermediate associated with transition state 1 (TS1) during proteolysis). In contrast, covalent interactions between a thiol and a nitrile form a thioimidate intermediate that mimics transition state 2 (TS2) of an enzymatic proteolytic event between a cysteine proteases and a substrate. Ménard and coworkers examined aldehyde and nitrile inhibitors of papain and found that the thioimidate intermediate engages the oxyanion hole interaction in a manner that more closely mimics the natural process of hydrolysis during proteolysis13. This may have consequences for both the binding affinity of nitrile-based cysteine proteases inhibitors and their ultimate resolution through hydrolysis of the thioimidate intermediate.
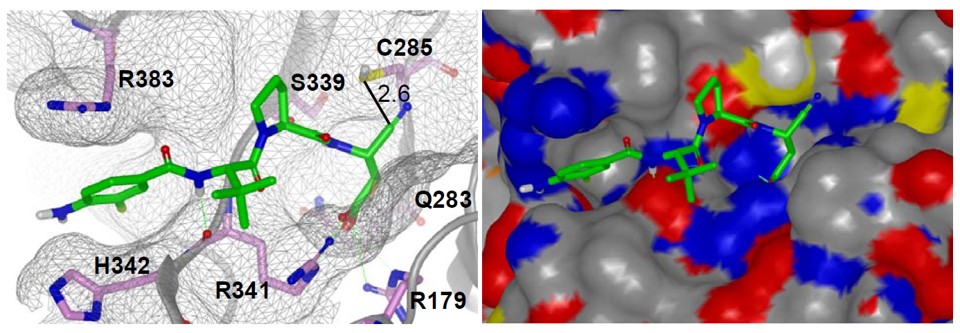
Figure 1. Molecular model (ribbon and space filling) of ML132 bound to Caspase 1.
References
- qHTS Assay for Allosteric/Competitive Inhibitors of Caspase-1: Summary
- Boxer MB, Quinn AM, Shen M, et al. A highly potent and selective caspase 1 inhibitor that utilizes a key 3-cyanopropanoic acid moiety. ChemMedChem. 2010;5(5):730-738. doi:10.1002/cmdc.200900531
- Martinon F, Tschopp J. Inflammatory caspases: linking an intracellular innate immune system to autoinflammatory diseases. Cell. 2004;117(5):561-574. doi:10.1016/j.cell.2004.05.004
- Braddock M, Quinn A. Targeting IL-1 in inflammatory disease: new opportunities for therapeutic intervention. Nat Rev Drug Discov. 2004;3(4):330-339. doi:10.1038/nrd1342
- Boxer MB, Shen M, Auld DS, et al. A small molecule inhibitor of Caspase 1. 2010 Feb 25 [Updated 2011 Mar 3]. In: Probe Reports from the NIH Molecular Libraries Program [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2010-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK56241/
- Romanowski MJ, Scheer JM, O'Brien T, McDowell RS. Crystal structures of a ligand-free and malonate-bound human caspase-1: implications for the mechanism of substrate binding. Structure. 2004;12(8):1361-1371. doi:10.1016/j.str.2004.05.010
- Wilson KP, Black JA, Thomson JA, et al. Structure and mechanism of interleukin-1 beta converting enzyme. Nature. 1994;370(6487):270-275. doi:10.1038/370270a0
- Okamoto Y, Anan H, Nakai E, et al. Peptide based interleukin-1 beta converting enzyme (ICE) inhibitors: synthesis, structure activity relationships and crystallographic study of the ICE-inhibitor complex. Chem Pharm Bull (Tokyo). 1999;47(1):11-21. doi:10.1248/cpb.47.11


