ML148 : HPGD (15-hydroxyprostaglandin dehydrogenase [NAD(+)]) Inhibitor
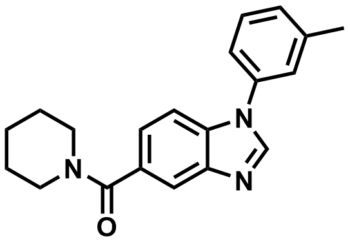
ML148
Target Name
15-hydroxyprostaglandin dehydrogenase [NAD(+)]
Target Alias
HPGD
Target Class
Dehydrogenase
Mechanism of Action
Inhibitor of HPGD
Biological / Disease Relevance
Pathways of Prostaglandins, Biological function and intracellular interactions of 15-PGDH
In vitro activity
HPGD bioassay (IC50)In vitro activity
HPGD Thermal shift assay (delta Tm)Target Information
15-hydroxyprostaglandin dehydrogenase (15-PGDH; HPGD) is the key enzyme for the inactivation of prostaglandins, and thus regulates processes such as inflammation or proliferation. The anabolic pathways of prostaglandins are well-characterized, especially with respect to regulation of the cyclooxygenase (COX) enzymes. In comparison, little is known about downstream events, including functional interaction of prostaglandin-processing and metabolizing enzymes, as well as the function of prostaglandin receptors. ML148 (CID-3243760) is a potent and competitive HPGD inhibitor that is selective within the dehydrogenase family.
Properties

ML148
NCGC00065167
| Physical & chemical properties | ||||
|---|---|---|---|---|
| Molecular Weight | 319.4 g/mol | |||
| Molecular Formula | C20H21N3O | |||
| cLogP | 3.8 | |||
| PSA | 38.1 | |||
| Storage | ||||
| Solubility | ||||
| CAS Number | 451496-96-1 | |||
SMILES:
CC1=CC(N2C=NC3=CC(C(N4CCCCC4)=O)=CC=C23)=CC=C1
InChI:
InChI=1S/C20H21N3O/c1-15-6-5-7-17(12-15)23-14-21-18-13-16(8-9-19(18)23)20(24)22-10-3-2-4-11-22/h5-9,12-14H,2-4,10-11H2,1H3
InChIKey:
YQNOQZALLOQMPY-UHFFFAOYSA-N
Activity
Summary activity statement /
ML148 (SID 87550718 ; CID 3243760) is a potent and selective inhibitor of HPDG. This probe has been tested in > 459 assays, and thus far, have only been active in HPGD-related assays performed at NCGC. ML148 is also found inactive against a related dehydrogenases. ML148 is the second chemotype of potent and selective inhibitor of HPDG.
In vitro activity - Selectivity Assay
| ML148 (IC50) | |
|---|---|
|
HPGD bioassay |
0.056 uM |
|
ALDH1A bioassay |
> 57 uM |
|
HADH2 bioassay |
> 57 uM |
|
HSD17beta4 bioassay |
> 57 uM |
Summary /
Selectivity of the ML148 is determined. Two structurally related dehydrogenases were chosen as suitable anti-targets for characterizing selectivity of the probe series: HADH2 and HSD17β4. The third anti-target is an ALDH1A1 which shares some homology with HPGD. ML147 is observed to be > 2000 fold, > 1000 fold, and > 1000 fold selective towards HPGD vs ALDH1A, HADH2, and HSD17beta4 respectively.
In vitro activity - ADME Profiling
| ML148 | |
|---|---|
|
aq. Kinetic sol. (PBS @ pH 7.4) |
3 uM |
|
Caco-2 (Papp 10^-6 m/s @ pH 7.4) |
15 |
|
Efflux ratio (B->A)/(A->B) |
0.53 |
|
Mouse Liver Microsome Stability (T1/2) |
< 10 min |
|
PBS-pH 7.4 Stability (% remaining after 48hr) |
100 |
|
Mouse Plasma Stability (% remaining after 48hr) |
98 |
Summary /
ADME data shows this compound is cell permeable and has an efflux ratio of ~0.53. This suggests that this probe would be suitable tool compound with which to investigate the effects of HPGD in a cellular context.
In vitro activity - Thermal Shift Assay
Summary /
ML148 is observed to have a strong stabilization (which is expressed as the shift in the transition midpoint temperature (ΔTm)) in the presence of presence of NAD+ or NADH. An IC50 of 7.9nM was determined for the re-synthesized compound, while the purified re-purchased dry powder gave an IC50 of 4.3nM.
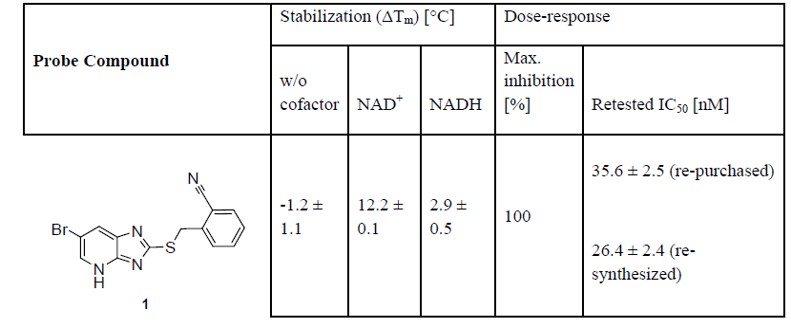
Table 1. ML148: Effect of probe compound on stability and activity of HPGD.
In vitro activity - Mechanism of Action
Summary /
Mechanism of action for ML148 is assessed using a kinetic characterization in experiments with titration of the 15-PGDH substrate PGE2 and of co-subtrate NAD, respectively. The plot generated suggests an uncompetative inhibition for ML148.
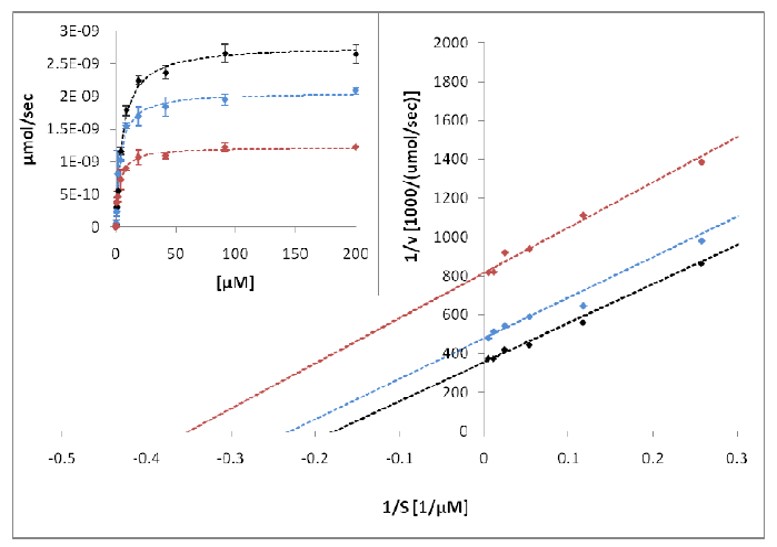
Figure 2. Mechanism-of-action studies of probe compound with respect to the substrate. Insets show the Michaelis-Menten graphs of 15-PGDH activity at increasing concentrations of PGE2, while the main graphs show plots of the data after Lineweaver and Burk. Dotted lines in both representations show fits to the Michaelis equation. Black represents no inhibitor, blue denotes 10nM probe compound, and red denotes 50nM probe compound.
References
- Jadhav A, Niesen FH, Schultz L, et al. Potent and selective inhibitors of NAD+-dependent 15-hydroxyprostaglandin dehydrogenase (HPGD) 2010 Mar 4 [Updated 2011 Mar 11]. In: Probe Reports from the NIH Molecular Libraries Program [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2010
- Probe Development Summary for Inhibitors of HPGD (15-Hydroxyprostaglandin Dehydrogenase)


