ML306 : Apoferritin (Apoferritin) Modulator

ML306
Target Name
Apoferritin
Target Alias
Apoferritin
Target Class
Metal Binding Protein
Mechanism of Action
Modulator of Apoferritin
Biological / Disease Relevance
General Anesthetic Mechanism, Anasthetics, Apoferritin
In vitro activity
Apoferritin (surrogate target) (IC50)In vitro activity
Isothermal titration calorimetry (ITC) (Kd)In vivo activity
Tadpole Immobility Assay (% Immobility)Target Information
General anesthetics are administered to more than 40 million patients in the U.S. each year. Their considerable side effects, including toxicity, subtle durable effects, and potential for interaction with the neurodegenerative diseases have prompted the search for novel general anesthetics that may act in a more specific manner and with fewer undesirable features. Evidence has shown that general anesthetics exert their functions by directly interacting with specific proteins, such as the GABAA receptor, a ligand-gated ion channel (LGIC). Photo-labeling studies have suggested that general anesthetics bind to LGIC transmembrane regions and allosterically regulating activity. Due to the limited availability of membrane proteins, especially hetero-oligomers like most GABAA receptors, detailed biochemical characterization has been difficult to perform. A surrogate approach was recently developed, where the iron-binding protein apoferritin, was demonstrated to possess not only strong binding capacity for many general anesthetics, but also to have a structural architecture highly resembling that of the GABAA receptor transmembrane region. Thus, identification of small molecules that bind to apoferritin, as detected by the use of a fluorescent reporter, can serve as a first step towards the identification of potent leads for novel general anesthetics. We adopted this apoferritin surrogate system to screen a ~351,000 member library using a highly miniaturized 1536-well based fluorescence assay in concentration-response mode. Using this approach, a novel general anesthetic class of 6-phenylpyridazin-3(2H)-ones was identified and developed, exemplified by ML306 which validated in two in vivo proof-of-concept models which utilized distinct probe delivery routes. The probe compound ML306 exhibits excellent pharmacokinetic properties and represents a useful tool molecule for the exploration of general anesthetic mechanisms and further pre-clinical development of safe anesthetics. ML306 is thus the first example of a candidate general anesthetic discovered through a rational high-throughput approach.
Properties

ML306
NCGC00140268
| Physical & chemical properties | ||||
|---|---|---|---|---|
| Molecular Weight | 347.4 g/mol | |||
| Molecular Formula | C21H21N3O2 | |||
| cLogP | 3.1 | |||
| PSA | 61.8 Ų | |||
| Storage | ||||
| Solubility | ||||
| CAS Number | ||||
SMILES:
CC1=CC=C(C2=NN(C(C=C2)=O)CC(NCCC3=CC=CC=C3)=O)C=C1
InChI:
1S/C21H21N3O2/c1-16-7-9-18(10-8-16)19-11-12-21(26)24(23-19)15-20(25)22-14-13-17-5-3-2-4-6-17/h2-12H,13-15H2,1H3,(H,22,25)
InChIKey:
VQUJWOQCTZQJTD-UHFFFAOYSA-N
Activity
Summary activity statement /
ML306 (SID 134419032; CID 5070704) represents a novel probe series which was identified in an HTS campaign using apoferrtin as a surrogate protein for GABAA and was subsequently optimized through extensive medicinal chemistry optimization. Importantly, ML306 possesses optimal pharmacokinetic properties for a potential general anesthetic and has demonstrated in vivo efficacy in two models (mouse and tadpole, which utilized distinct probe delivery routes) without exhibiting significant toxicity at the administered doses. The probe is useful to further evaluate the surrogate approach through the use of high throughput assays for the discovery of novel compounds which exhibit anesthetic activity. Given ML306’s in vivo profile, it can be used to validate new anesthesia models such as those utilized in medium throughput zebrafish screening (studies in progress). The initial profiling of ML306 presented in this report presents negative data that helps eliminate candidate targets, thus informing future mechanism of action studies, and its deviation from the Meyer-Overton rule make it an interesting candidate for studies in the field of general anesthetics. Finally, given its medicinal chemistry optimization potential, ML306 serves as a starting point for the pre-clinical development of a novel class of general anesthetics.
In vitro activity - Selectivity and Cytotoxicity Assay
| Bioassay | ML306 (IC50) |
|---|---|
|
Apoferritin (Surrogate target) |
3.04 uM |
|
Tadpole Toxicity (Anti-Target) |
0% toxicity at 10 uM |
Summary /
ML306 is obsereved to be active against the Apoferritin assay and in the tadpole immobilization assay with no apparent toxic effect.
In vitro activity - Isothermal Titration Calorimetry (ITC)
Summary /
In order to further triage primary hits identified from the qHTS screen, ITC was utilized to evaluate direct binding signatures between filtered compounds and apoferritin and provide a complete thermodynamic characterization of selected compounds. ITC was performed on a MicroCal VP-ITC instrument (http://microcal.com/, Northampton, MA). Compound powders were obtained directly from commercial sources, and ~1 mM solution was prepared in the qHTS assay buffer without DMSO. This was achieved by vigorous shaking and repetitive sonication of the solution, followed by filtration through 0.2 μm PTFE syringe filters to remove compound aggregates. Concentrations were then determined using absorption spectroscopy based on standards prepared in organic solvents. The ITC sample cell (1.43 mL) was loaded with apoferritin (also in the qHTS assay buffer; the reference cell contained water) while the syringe was loaded with compounds. Each ligand was titrated into the sample cell from the syringe containing 286 μL of ligand solution. Additional titrations were performed for correction of heat of dilution, including buffer into buffer, buffer into protein and compound into buffer. At least duplicate experiments were performed at room temperature, and data were corrected and fitted to single class binding models using Origin Software.
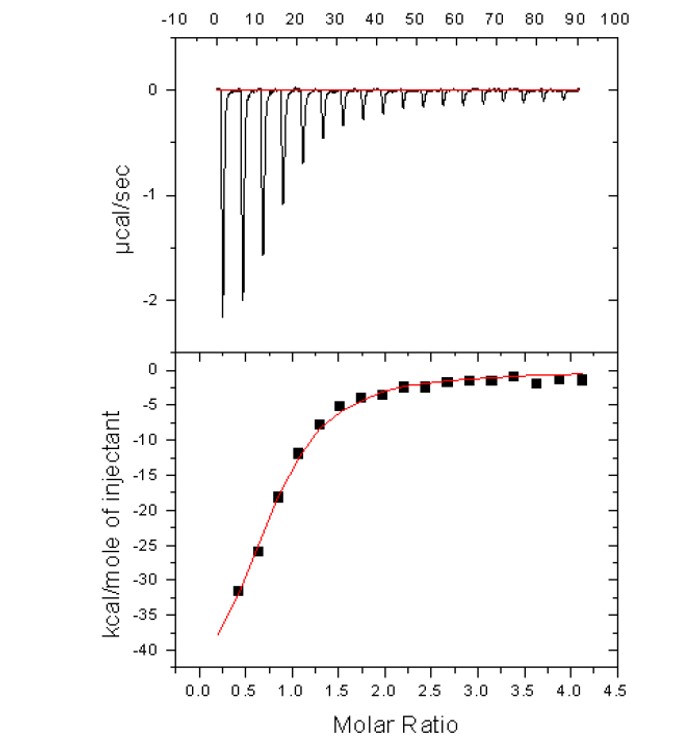
Figure 1. Isothermal Titration Calorimetry (ITC) of the HSAF interaction with ML306 (KD = 8.2 μM).
In Vivo Activity - Mouse model loss of righting reflex (LORR)
Summary /
ML306 produced loss of righting reflex (LORR) in less than 20 seconds in mice, with durations that depended on dose. An 80 mg/kg injection iv produced an average time of 73 ± 45 seconds until mice were able to right themselves (n = 6). The response to this dose varied between 0 seconds (misplaced injection) and 256 seconds. Conversely, 100 mg/kg intravenous (IV) administration of ML306 produced LORR in all animals (n = 3), with LORR ranging from 140 to 362 seconds. 40 mg/kg IV administration was determined to be ineffective in all mice tested (n = 4). Control injections with the vehicle solution ranged from 1304 mg HP-β-CD/kg body mass to 2557 mg/kg and produced no observable effects (n = 4). The latter dose approximates published data that suggests decreased activity and breathing irregularities following an intravenous injection of 2250 mg/kg in rats (Gould 2005). Upon recovery of righting reflex, the mice were subdued, exhibited slow exploratory behavior, and did not attempt to avoid the handler. Apparently normal behavior was observed within ten minutes following the injection. No apparent toxicity or residual effect of the injection was noted out to two weeks. Two weeks post injection, each mouse had gained a minimum of 0.4 grams of body mass, not different from the vehicle injected controls.
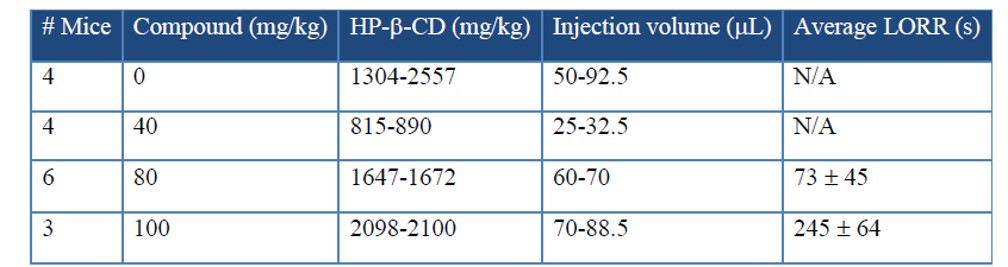
Table 1. ML306 In vivo loss of righting reflex (LORR) studies. Standard error shown for LORR time. The molecular weight of the ML306 (461 g/mol) salt is ~2.6 times that of propofol (178 g/mol). For comparison, a 20 mg/kg injection of propofol suspended in intralipid induces LORR for 216 ± 25 seconds. 100 mg/kg ML306 is equal to 0.217 mmol/kg, whereas 20 mg/kg propofol is equal to 0.112 mmol/kg.
In vivo - Pharmacokinetic Profiling
Summary /
All experiments were conducted at Pharmaron Inc. (a) Represents the kinetic solubility in PBS buffer (pH = 7.4). (b) Represents the stability in the presence of NADPH. The probe compound showed no degradation without NADPH present over a 1 hr period. (c) Dextromethorphan was used as the substrate. (d) Midazolam was used as the substrate.
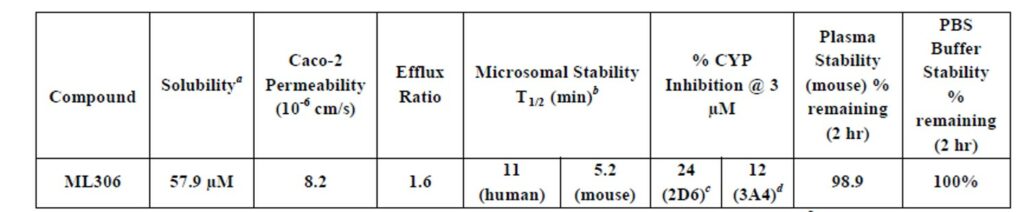
Table 2. ADME profile for ML306.
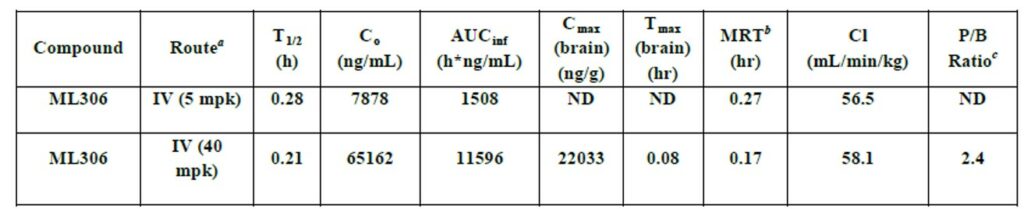
Table 3. In vivo PK (mouse) at 40 mpk IV and 5 mpk IV. All experiments were conducted at Pharmaron Inc. with male CD1 mice (6-8 weeks of age). Data was collected in triplicate at 8 time points over a 24 hr period. (a) Both formulated as a solution [HP-β-CD in saline (1 g/mL)]. (b) Mean residence time (the time for elimination of 63.2% of the IV dose). (c) Plasma to brain ratio [AUClast(plasma)/ AUClast(brain)].
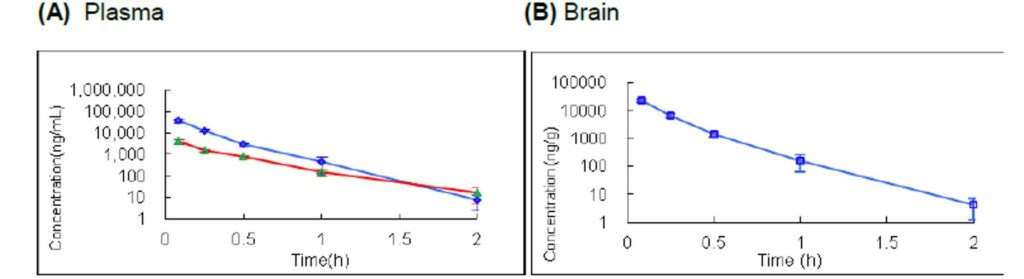
Figure 2. (A) PK plasma profile for ML306. CD1 Mice IV (40 mpk (blue) and 5 mpk (red)) dosed as a solution with n = 3 for each time point. (b) PK brain profile for ML306. CD1 Mice IV (40 mpk) dosed as a solution with n = 3 for each time point.
In vitro activity - Mechanism of Action
Summary /
Propofol is, in part, thought to produce its effect by interacting with inhibitory cys-loop ligand-gated ion channels, although there exists evidence that it also has effects on other ion channels, such as the voltage gated cation channels. Although our surrogate screen is based an ability of apoferritin to mimic GABAergic allosteric binding, apoferritin clearly cannot mimic the ion channel activity of the GABAA receptor. This activity, however, is simply not amenable to high throughput screening as it requires an electrophysiology rig. One assay that more specifically evaluates GABAAR allosteric binding activity is the ability of compounds to enhance the binding of radiolabeled flunitrazepam. Preliminary results from Eckenhoff Lab show GABAergic activity for several members of the probe series. It is conceivable that a portion of the sedative action of members of the probe series is due to enhancement of GABAergic activity, as the original surrogate was designed to reveal. Although our preliminary results showed that the probe ML306 was not active in the radiolabeled flunitrazepam assay, the fact that at the same time a number of its analogues did show activity comparable to propofol (data not shown), indicates that additional studies using the radiolabeled flunitrazepam, as well as alternative assay platforms to interrogate GABAergic activity, are warranted.
ITC against apoferritin was used only to confirm the qHTS results, since the latter may be contaminated by various optical effects, such as inner filter, FRET and so forth. ITC proved very difficult as the compounds were not available in sufficient mass in powder form to conduct several runs (which, given the expected micromolar KD, required the application of very high compound concentrations, beyond those supported by their aqueous solubilities) in the absence of DMSO. Cosolvents such as DMSO produce large heats of dilution which can obscure the often smaller heats of bimolecular binding interactions. Thus, after several attempts, the ITC confirmation of qHTS, even of the subsets, was abandoned in favor of the phenotype assays. However, ML306 and the original hit CID-3244374 did prduce a binding isotherm in apoferritin ITC experiments, yielding KD’s of 8.2 μM and 4.2 μM, respectively.
In vitro - Pharmacological Profiling
Summary /
ML306 was submitted to the National Institute of Mental Health’s Psychoactive Drug Screening Program (PDSP) G protein-coupled receptor (GPCR) Panel, and the results were represented as a heatmap. ML306 along with propofol were tested at 1 μM against a panel of 149 GPCRs. Compounds with < 4-fold stimulation at 1 μM were considered uninteresting for further potency determination. Internal positive controls were used in the panel (data not shown). Surprisingly, both ML306 and propofol were very clean with no significant activity against any of the tested GPCRs. Profiling against ligand gated voltage cannels and other selected receptors are ongoing. We were encouraged by this profile as it seems to indicate that the in vivo effect is not simply a result of promiscuous GPCR activity, however it also means that additional profiling must be done to help identify the target(s). It should be noted that such a task is a lofty goal given that researchers do not yet fully understand how known anesthetics work despite being studied extensively for decades.
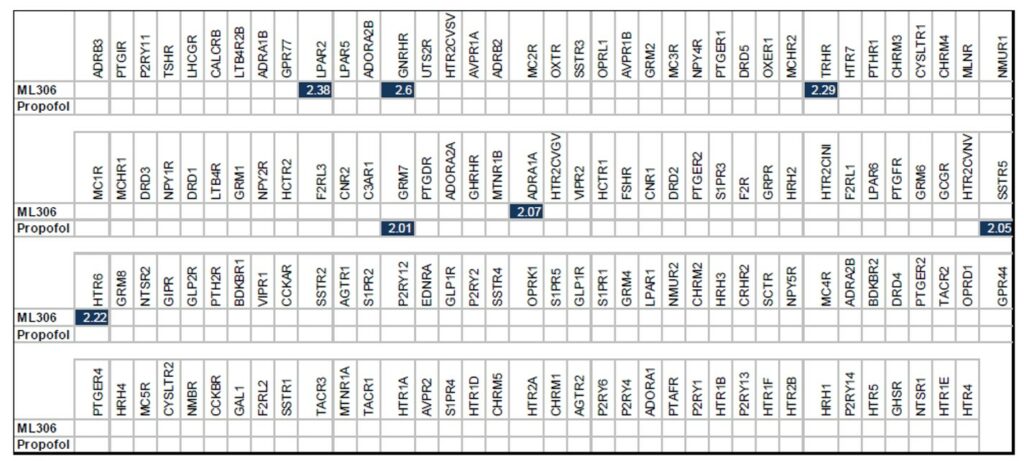
Table 4. PDSP GPCR Profiling of ML306 and propofol tested at 1 μM concentration. White squares represent inactivity while blue squares represent fold stimulation at 1 μM.
References
- Probe Development Summary for Identification of Novel General Anesthetics
- Rai G, Bu W, Lea WA, et al. Discovery of Novel General Anesthetics Using Apoferritin as a Surrogate System. In: Probe Reports from the NIH Molecular Libraries Program. Bethesda (MD): National Center for Biotechnology Information (US); April 16, 2012.
- Gould S, Scott RC. 2-Hydroxypropyl-beta-cyclodextrin (HP-beta-CD): a toxicology review. Food Chem Toxicol. 2005;43(10):1451-1459. doi:10.1016/j.fct.2005.03.007


