ML372 : SMN2 (Survival Motor Neuron 2) Modulator
ML372
Target Name
Survival Motor Neuron 2
Target Alias
SMN2
Target Class
RNA Splicing Factor
Mechanism of Action
Modulator of SMN2
Biological / Disease Relevance
Spinal Muscular Atrophy (SMA), Splicing Modulator, Gene splicing, SMN2 splicing
In vitro activity
SMN expression - patient fibroblast (EC50)In vivo activity (SMNdelta7 SMA Mice - 50 mg/kg, 2x/day)
Survival StudiesIn vivo activity (SMNdelta7 SMA Mice - 50 mg/kg, 2x/day)
Righting Time StudiesIn vivo activity (SMNdelta7 SMA Mice - 50 mg/kg, 2x/day)
Body Weight StudiesTarget Information
Spinal muscular atrophy (SMA) is an autosomal recessive neurodegenerative disease and the most common inherited cause of infant mortality. SMA, with a carrier frequency of ∼1:40, affects ∼ 1:8,000 births. In severe cases, death usually ensues within the first two years of life. There are currently no therapeutic treatments for SMA other than supportive care. At a molecular level, SMA is caused by insufficient levels of the survival motor neuron (SMN) protein. Our group recently reported on the discovery and optimization of a new series of small molecules, represented by ML372, with good potency, pharmacokinetics, tolerance, and CNS penetration that are able to increase levels of SMN protein in several model cell lines (Xiao 2011). Herein, we characterize the ability of ML372 to increase SMN protein levels in vivo, restore motor function, and prolong survival of SMNΔ7 SMA Mice (Burnett 2013, Abera 2016).
Properties

ML372
NCGC00187872
| Physical & chemical properties | ||||
|---|---|---|---|---|
| Molecular Weight | 360.4 g/mol | |||
| Molecular Formula | C18H20N2O4S | |||
| cLogP | 3.2 | |||
| PSA | 100 Ų | |||
| Storage | ||||
| Solubility | ||||
| CAS Number | 1331745-61-9 | |||
SMILES:
OC(C1CCN(C2=NC=C(C3=CC4=C(C=C3)OCCCO4)S2)CC1)=O
InChI:
1S/C18H20N2O4S/c21-17(22)12-4-6-20(7-5-12)18-19-11-16(25-18)13-2-3-14-15(10-13)24-9-1-8-23-14/h2-3,10-12H,1,4-9H2,(H,21,22)
InChIKey:
HAVNRFQWAXTDTI-UHFFFAOYSA-N
Activity
Summary activity statement /
ML372 (CID 46907666; SID 99367980) is a brain-penetrant analog from a series of aryl-thiazol-piperidines which increase SMN protein levels in SMA-cells. This series was discovered in a high-throughput screen for modulators of full length SMN protein expression using an SMN2-reporter assay. ML372’s activity as a novel SMN modulator was confirmed by western blot analysis and gem count assays using SMA patient fibroblasts. Preliminary pharmacokinetic experiments also demonstrated that ML372 was able to achieve significant brain exposure in mice. This report demonstrates the ability of ML372 to augment SMN protein in vivo, rescue motor function, and alter the lifespan in SMNΔ7 SMA mice. These results validate ML372 as a novel SMN modulator and establish it as an effective proof-of concept molecule that can be advanced as preclinical candidate for the treatment of SMA.
Cellular activity - SMN protein expression (Western blot)
Summary /
The activity of compounds including ML372 were evaluated by western blot analysis and gem count assays using SMA patient fibroblasts (Figure 1), showing increments of SMN levels and gem numbers at concentrations as low as double digit nanomolar and above. ML372 increase levels of SMN protein in patient cell lines as concentrations as low as 37 nM, clearly demonstrating that the elevation of signal in the reporter assay was mainly due to increments in SMN levels. ML372 showed a dose-dependent trend between 37 nM to 1 μM concentration and a slightly decrease of protein level at 3 μM. In the gem count assay, primary human fibroblasts were treated with increasing concentrations of ML372 for 3 days and the number of gems per 100 nuclei were examined (Figure 1B). ML372 also showed an 80% increase of the gem numbers between 37 nM to 1 μM concentrations and slightly decreased at 3 μM.
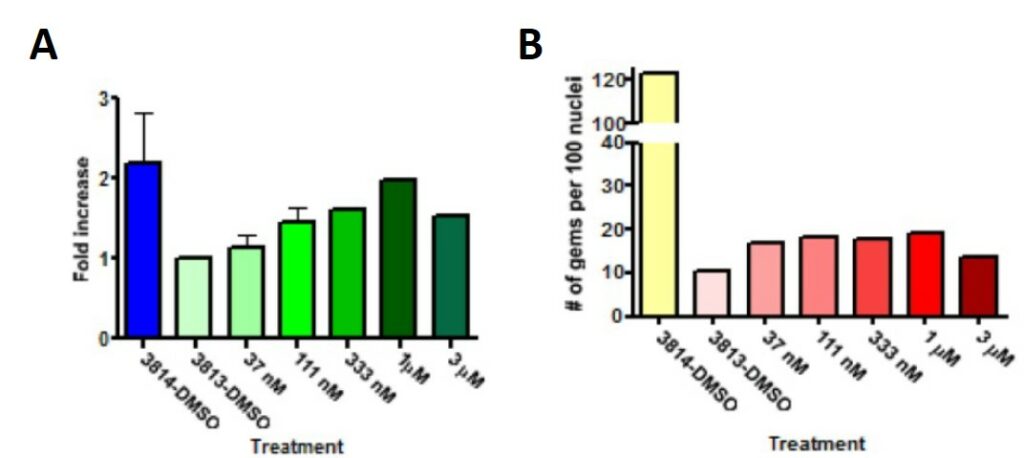
Figure 1. (A). Quantification of western blot of SMN levels after treatment with ML372 at different concentrations, as indicated. (B) Number of gems per 100 nuclei after treatment with ML372 at different concentrations, as indicated. In the western blot assay, primary human fibroblast lysates from carrier (3814: SMN1+/−; SMN2+/+) and SMA (3813: SMN1−/−; SMN2+/+) cells were blotted with antibodies to SMN and α-tubulin. Cells were treated for 48 hr with increasing concentrations of ML372. Fold increase of SMN protein was calculated in relation to DMSO-treated 3813 and normalized to tubulin levels (Figure 1A).
In vivo activity - Pharmacokinetic (PK) study
Summary /
In consideration of a combination of potency, efficacy, permeability, physical and metabolic stability, ML372 was chosen for mouse pharmacokinetic (PK) study to evaluate the central nervous system (CNS) penetration (Table 1). The plasma and brain concentration of ML372 in male Swiss Albino mice after a single oral gavage administration at a dose of 30 mg/kg were measured. As shown in Table 1 the brain to plasma ratio of ML372 in male Swiss Albino mice was found to be 0.028. In addition, ML372 possessed a reasonable long half-life in brain (T = 13.7 hours) as well as in plasma (T = 11.2 hours). Despite its high plasma protein binding (94.9%) for ML372, its concentration in brain reached 1.23 μmol/kg (C ) in 15 minutes and a level above its minimal effective concentration of 0.037 μM in SMA fibroblasts for over 24 hours. In addition, no behavioral disturbance was observed in animals administered with ML372 throughout the study period.
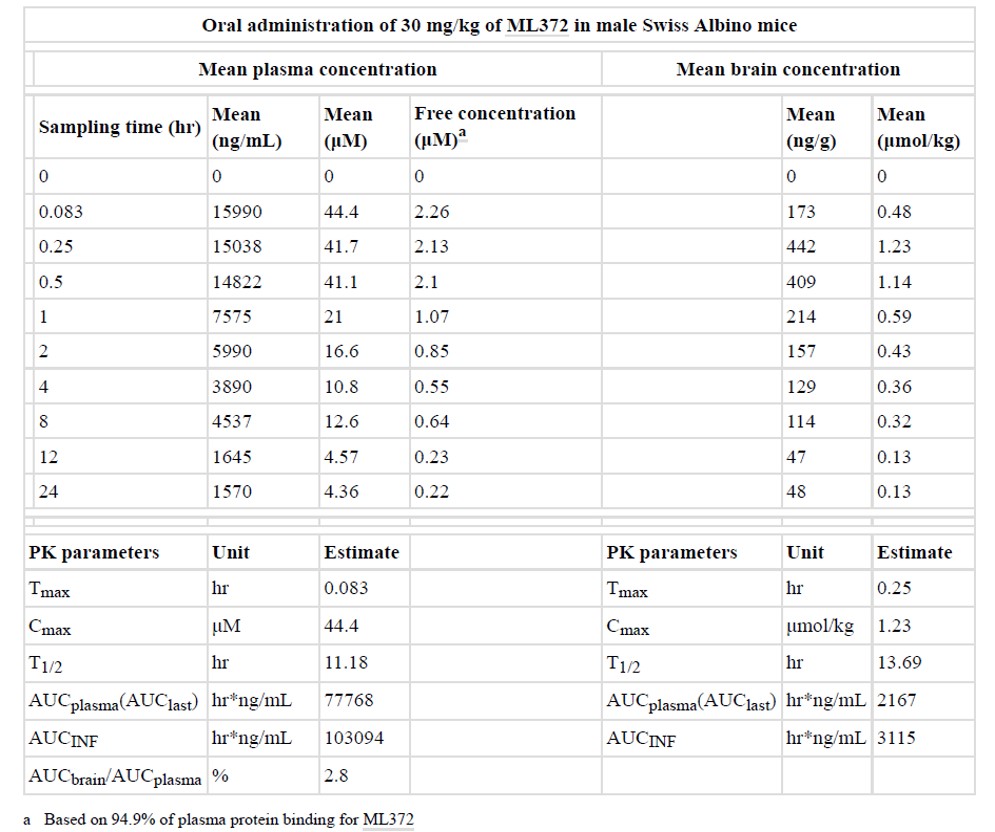
Table 1. Summary of mouse in vivo pharmacokinetics of ML372.
In vitro activity - Comparison to prior art
Summary /
SMA has become a focus of therapeutic development activity, and there are 13 novel therapeutic programs for SMA in various stages of preclinical and clinical research (Figure 2) which leverage a wide variety of approaches including small-molecule regulators, gene therapy, stem cell therapy, and anti-sense nucleotide therapy. Among these programs, there are now three novel programs actively being testing in clinical trials for SMA: Trophos has taken Olesoxime into Phase II clinical trials, Repligen Corporation has a quinazoline currently in Phase I clinical trials, and Isis Pharmaceuticals and Biogen Idec are also in Phase I clinical trials using their anti-sense nucleotide therapy approach. A variety of other preclinical programs are known to be developing clinical candidates, but their templates and mode of actions are not known. Table 2 summarizes their corresponding mechanism of action and biological responses. Although Olesoxime is in a clinical stage to assess the efficacy and the safety of olesoxime in SMA type 2 or type 3 non ambulant patients aged 3-25 years, the detailed data from in vitro and in vivo studies are not publically disclosed. Trophos briefly stated that Olesoxime has been shown to be active in multiple preclinical neurodegeneration models, including the NSE-Cre F7/F7 model of SMA. Regarding the tetracycline PTK-SMA1, in general one of the major bottlenecks of these type of compounds is that they usually do not cross the Blood Brain Barrier (BBB) which probably excludes this molecule for the treatment of SMA. Quinazoline increases SMN protein expression and the mean lifespan of SMN delta 7 mice. However, it requires pre-birth treatment to have a reasonable effect, making this approach impractical for the treatment of human SMA patients because there is no pre-birth screening for SMA. Indoprofen indeed has shown recovery of SMN protein levels, however, it has severe limitations, such as solubility issues and being unable to improve animal body weight and survival. Although significant increases in SMN protein levels were observed in a moderate SMA mouse model treated with an Indoprofen analog, ALB 111 (structure not known), for 15 days, when tested in a more severe mouse model of SMA, the benefits were more limited. Subsequent analysis suggests that the main hurdle with ALB-111 is its limited solubility which limits proper formulation. NINDS is in the stage of close-out of this SMA project in June 2012. Herein, we show that the NCGC probe ML372, optimized from a series of analogs, addressed these issues.

Table 2. Comparison of ML372 to small molecule prior art.
References
- Quantitative High-Throughput Screen for Enhancers of SMN2 Splice Variant Expression: Summary
- Xiao J, Marugan JJ, Zheng W, et al. Discovery, synthesis, and biological evaluation of novel SMN protein modulators. J Med Chem. 2011;54(18):6215-6233. doi:10.1021/jm200497t
- Burnett BG, Xiao J, Southall N, et al. SMN Modulator ML372 Increases SMN Protein Abundance, Body Weight, Lifespan, and Rescues Motor Function in SMNΔ7 SMA Mice. 2013 Dec 15 [Updated 2015 Feb 11]. In: Probe Reports from the NIH Molecular Libraries Program [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2010
- Extended Characterization of SMN2 Inhibitors: Survival Studies
- Extended Characterization of SMN2 Inhibitors: Righting Time Studies
- Extended Characterization of SMN2 Inhibitors: Body Weight Studies
- Abera MB, Xiao J, Nofziger J, et al. ML372 blocks SMN ubiquitination and improves spinal muscular atrophy pathology in mice. JCI Insight. 2016;1(19):e88427. Published 2016 Nov 17. doi:10.1172/jci.insight.88427


