ML355 : ALOX12 (Arachidonate 12-lipoxygenase) Inhibitor
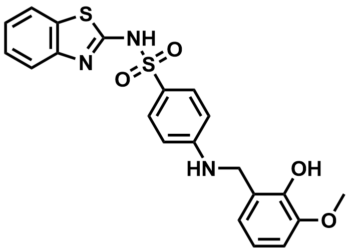
ML355
Target Name
Arachidonate 12-lipoxygenase
Target Alias
ALOX12
Target Class
Oxygenase
Mechanism of Action
Inhibitor of ALOX12
Biological / Disease Relevance
Human lipoxygenases (LOXs), Fatty Acid Oxidation, Cancer Biology
In vitro activity
12-LOX (IC50)Target Information
Human lipoxygenases (LOXs) are enzymes involved in catalyzing the oxidation of polyunsaturated fatty acids to provide the corresponding bioactive hydroxyeicosatetraenoic acid (HETE) metabolites as the end product (Yamamato 1997, Solomon 1997, Brash 1999). These eicosanoid signaling molecules are involved in a number of physiologic responses such as platelet aggregation, inflammation and cell proliferation (Kuhn 2006, Nie 2000, Yeung 2012). As a result, modulation of these responses through the inhibition of the lipoxygenase enzymes is of great interest. Our group has particular interest in platelet-type 12-(S)-LOX (12-LOX) because of its demonstrated role in skin diseases, diabetes, platelet hemostasis, thrombosis and cancer (Catalano 2005, Thomas 2010). However, despite the potential of 12-LOX as a therapeutic target, few potent and selective inhibitors have been reported. The lack of high quality 12-LOX inhibitors prompted us to initiate a high-throughput screening campaign as part of the MLPCN program which ultimately led to the discovery of ML127. While potent and selective, ML127 demonstrated limited tolerance for structural modifications, which hampered continued medicinal chemistry efforts thus a continued discovery efforts to develop additional novel inhibitors of 12-LOX is needed. Herein, we report the identification and medicinal chemistry optimization of an unrelated, second chemotype, ML355, which displays nanomolar potency against 12-LOX and excellent selectivity over related lipoxygenases and cyclooxygenases. ML355 has favorable absorption, distribution, metabolism, and excretion (ADME) properties, inhibits PAR-4 induced aggregation and calcium mobilization in human platelets, and reduces 12-HETE in mouse/human beta cells suggesting its potential utility in animal models for antiplatelet therapy and diabetes (Luci 2010).
Properties

ML355
NCGC00263773
| Physical & chemical properties | ||||
|---|---|---|---|---|
| Molecular Weight | 441.5 g/mol | |||
| Molecular Formula | C21H19N3O4S2 | |||
| cLogP | 4.3 | |||
| PSA | 137 Ų | |||
| Storage | ||||
| Solubility | ||||
| CAS Number | 1532593-30-8 | |||
SMILES:
COC1=C(C(CNC2=CC=C(S(=O)(NC3=NC4=C(C=CC=C4)S3)=O)C=C2)=CC=C1)O
InChI:
1S/C21H19N3O4S2/c1-28-18-7-4-5-14(20(18)25)13-22-15-9-11-16(12-10-15)30(26,27)24-21-23-17-6-2-3-8-19(17)29-21/h2-12,22,25H,13H2,1H3,(H,23,24)
InChIKey:
OWHBVKBNNRYMIN-UHFFFAOYSA-N
Activity
Summary activity statement /
12-LOX has been implicated in the pathophysiology of a variety of diseases including arterial thrombosis and diabetes (T1D and T2D). Thus, targeted inhibition of 12-LOX has been proposed as a therapeutic strategy to mitigate the effects of these diseases by ultimately reducing the production of the bioactive metabolite 12-HETE. The ML355 (SID 160844040; CID 70701426) probe displays potent and selective inhibition of 12-LOX in vitro and demonstrated good activity in cell-based assays. ML355 has been shown to decrease calcium mobilization and PAR-4 induced platelet aggregation in patient derived human platelets and to significantly inhibit AA/IONO-induced 12-HETE in mouse BTC3 cells and human islets. Hence the ML355 probe can be used by researchers to interrogate the role of 12-LOX in both diabetes and antiplatelet in vivo models via pharmacological inhibition. Moreover, ML355 has shown favorable ADME properties enabling the scientific community to explore its potential therapeutic applications for other diseases where the 12-LOX role is crucial.
In vitro activity - Selectivity and Cytotoxicity Assay
Summary /
Selective profiling of ML355 and selected analogs showed inactivity of ML355 against human reticulocyte 15-lipoxygenase-1 (15-LOX-1), human epithelial 15-lipoxygenase-2 (15 LOX-2) and arachidonate 5-lipoxygenase (5-LOX) but good inhibition against human platelet 12-lipoxygenase (12-LOX) with 0.29 μM potency.
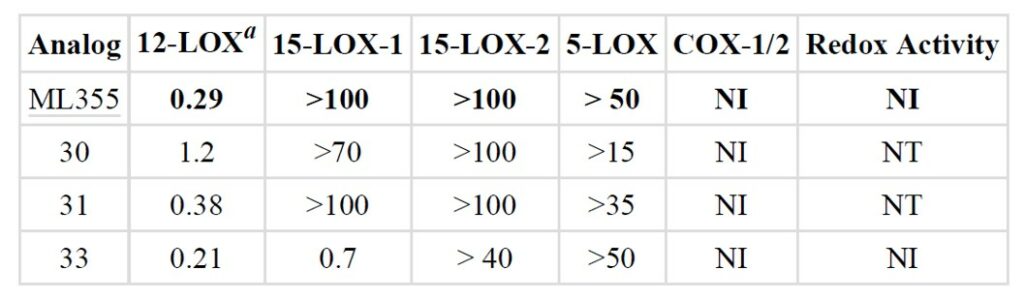
Table 1. Selectivity profiling of ML355 and other top compounds. NT = not tested while NI = No inhibition.
In vitro activity - Comparisson to Prior art
Summary /
Previously reported inhibitors of 12-LOX (Table 2) such as baicalein (Svensson 2013) and nor-dihydroguairetic acid (NDGA) (Whitman 2002), “bromo-phenols” or “pyrazole derivatives” all possess several liabilities. These compounds are not only less potent, selective, but are also not easily amendable to further optimization. Our previously described 12-LOX inhibitor (ML127) demonstrates potent inhibition (<500 nM) and excellent selectivity but is not very tolerant of structural modifications (Kenyon 2011). Few regions of the molecule are amendable for optimization limiting available avenues for medicinal chemistry efforts. In addition, while the 8-HQ scaffold of ML127 has not yet emerged as a liability for the series, the known promiscuous metal chelation for related compounds of that type suggested one should proceed with caution. In comparison, the new ML355 probe series demonstrated potent (290 nM) activity towards 12-LOX and excellent selectivity against related enzymes 15-LOX-1, 5-LOX, 15-LOX-2, and COX ½ (Table 1). Additionally, this chemotype is structurally distinct from all previously reported inhibitors (including ML127) and it possesses a very drug-like scaffold. This series is readily amendable to structural modifications and displays clear and tractable SAR. Most importantly, ML355 exhibits a favorable in vitro ADME and in vivo PK profile with activity in disease relevant cell-based systems like diabetes through 12-HETE reduction in β-cells.
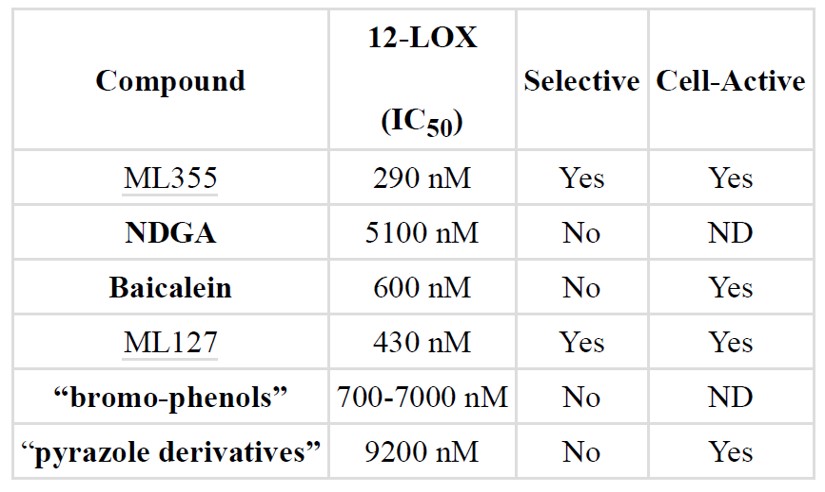
Table 2. Comparison of ML355 to previously identified 12-LOX inhibitors. ND = Not Determined.
Cellular activity - Mouse Beta cell (12-HETE Inhibition) functional assay
Summary /
Mouse beta cells (BTC3) were treated with arachidonic acid and calcium ionophore (AA/IONO) alone or in the presence of ML355. Result showed inhibition of 12-HETE expression by the ML355 probe.
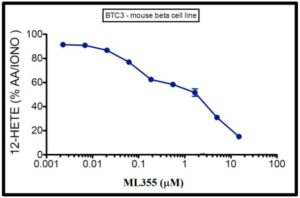
Figure 1. Dose dependent inhibition of stimulated 12-HETE by ML355. Graphed are the levels of 12-HETE expressed as a percentage of that detected in cells stimulated with AA/IONO alone. 12-HETE was measured by ELISA.
Cellular activity - Human islet (12-HETE Inhibition) functional assay
Summary /
Result showed inhibition of the of the 12-HETE expression on stimulated human islets upon treatment of ML355.

Figure 2. Histogram of 12-HETE expression (measured by ELISA) in human primary donor islets stimulated with Arachidonic acid and calcium ionophore (AA/IONO) alone or in the presence of 10 μM of ML355.
In vitro and vivo activity - ADME Profiling and PK Studies
Summary /
ML355 demonstrated excellent microsomal stability with both rat (T1/2 >30 minutes) and mouse (T1/2 >300 minutes) and was found to be stable to mouse plasma over a 2 hour period (100% remaining). ML355 showed no degradation over various aqueous buffers (pH 2-9) and was stable to 5 mM glutathione suggesting excellent stability. One remaining liability is the aqueous solubility which is <5 μM, however improved solubility is observed in the assay buffer (qualitative analysis). ML355 showed moderate permeability in the Caco-2 assay (1.5 × 10 cm/s) and does not appear to be a substrate for Pgp given the efflux ratio of <2. In vivo PK studies where ML355 was administered as a solution via IV (3mpk) and PO (30mpk) demonstrated that ML355 is orally bioavailable (%F = 20) with good half-life (T1/2 = 2.9 hours). At 30 mpk dosing, ML355 achieves a Cmax of over 135 times the in vitro IC50 and remains over IC50 value for over 12 hours. The compound has low clearance (3.4 mL/min/kg) and good overall exposure (AUCinf ) of 38 μM. Although, the volume of distribution (V ) observed was low (0.55 L/kg), the rest of the PK profiling results suggested a reasonable distribution between tissue and blood. These profiling results provided informative data necessary to develop an appropriate dosing regimen for future in vivo studies.
References
- Probe Development Summary of Inhibitors of 12-hLO (12-human lipoxygenase)
- Luci D, Jameson JB II, Yasgar A, et al. Discovery of ML355, a Potent and Selective Inhibitor of Human 12-Lipoxygenase. 2013 Apr 12 [Updated 2014 Sep 18]. In: Probe Reports from the NIH Molecular Libraries Program [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2010
- Yamamoto S, Suzuki H, Ueda N. Arachidonate 12-lipoxygenases. Prog Lipid Res. 1997;36(1):23-41. doi:10.1016/s0163-7827(97)00002-7
- Solomon EI, Zhou J, Neese F, Pavel EG. New insights from spectroscopy into the structure/function relationships of lipoxygenases. Chem Biol. 1997;4(11):795-808. doi:10.1016/s1074-5521(97)90113-7
- Brash AR. Lipoxygenases: occurrence, functions, catalysis, and acquisition of substrate. J Biol Chem. 1999;274(34):23679-23682. doi:10.1074/jbc.274.34.23679
- Kühn H, O'Donnell VB. Inflammation and immune regulation by 12/15-lipoxygenases. Prog Lipid Res. 2006;45(4):334-356. doi:10.1016/j.plipres.2006.02.003
- Nie D, Tang K, Diglio C, Honn KV. Eicosanoid regulation of angiogenesis: role of endothelial arachidonate 12-lipoxygenase. Blood. 2000;95(7):2304-2311
- Yeung J, Apopa PL, Vesci J, et al. Protein kinase C regulation of 12-lipoxygenase-mediated human platelet activation. Mol Pharmacol. 2012;81(3):420-430. doi:10.1124/mol.111.075630
- Catalano A, Procopio A. New aspects on the role of lipoxygenases in cancer progression. Histol Histopathol. 2005;20(3):969-975. doi:10.14670/HH-20.969
- Thomas CP, Morgan LT, Maskrey BH, et al. Phospholipid-esterified eicosanoids are generated in agonist-activated human platelets and enhance tissue factor-dependent thrombin generation. J Biol Chem. 2010;285(10):6891-6903. doi:10.1074/jbc.M109.078428
- Svensson Holm AC, Grenegård M, Ollinger K, Lindström EG. Inhibition of 12-lipoxygenase reduces platelet activation and prevents their mitogenic function. Platelets. 2014;25(2):111-117. doi:10.3109/09537104.2013.783688
- Whitman S, Gezginci M, Timmermann BN, Holman TR. Structure-activity relationship studies of nordihydroguaiaretic acid inhibitors toward soybean, 12-human, and 15-human lipoxygenase. J Med Chem. 2002;45(12):2659-2661. doi:10.1021/jm0201262
- Kenyon V, Rai G, Jadhav A, et al. Discovery of potent and selective inhibitors of human platelet-type 12- lipoxygenase. J Med Chem. 2011;54(15):5485-5497. doi:10.1021/jm2005089


