ML285 : PKM (Pyruvate Kinase M2) Activator
ML285
Target Name
Pyruvate Kinase M2
Target Alias
PKM
Target Class
Kinase
Mechanism of Action
Activator of PKM
Biological / Disease Relevance
Tumor cell growth and proliferation, Glucose metabolism
In vitro activity
hPyk-M2 Bioassay (IC50)In vitro activity
LDH assay (IC50)Target Information
The ability of all cells to regulate levels of reactive oxygen species (ROS) is vital for controlling many aspects of proliferation and survival and we have discovered that pyruvate kinase M2 (PKM2) is important for cancer cell biology. PKM2 is directly oxidized on Cys 358 to inhibit its catalytic activity, which allows for diversion of glucose-6-phosphate into the pentose phosphate pathway. This, in turn, allows the synthesis of NADPH, which is critical for generating reduced glutathione, necessary for ROS detoxification. In a cellular context, our PKM2 activator, ML285 protects the enzyme from oxidation by ROS and results in sensitization to oxidative stress and increased apoptosis.
Properties

ML285
NCGC00181061
| Physical & chemical properties | ||||
|---|---|---|---|---|
| Molecular Weight | 460.5 g/mol | |||
| Molecular Formula | C18H18F2N2O6S2 | |||
| cLogP | 1.6 | |||
| PSA | 110 Ų | |||
| Storage | ||||
| Solubility | ||||
| CAS Number | ||||
SMILES:
FC1=C(S(=O)(N2CCN(S(=O)(C3=CC4=C(OCCO4)C=C3)=O)CC2)=O)C(F)=CC=C1
InChI:
1S/C18H18F2N2O6S2/c19-14-2-1-3-15(20)18(14)30(25,26)22-8-6-21(7-9-22)29(23,24)13-4-5-16-17(12-13)28-11-10-27-16/h1-5,12H,6-11H2
InChIKey:
SHWNKRPMUBFWKE-UHFFFAOYSA-N
Activity
Summary activity statement /
ML285 ((SID 85267861; CID 25210493) is a potent activator of PKM2 in both biochemical (AC = 82 nM) and cell-based assays with high selectivity over PKM1, PKR and PKL. The compound was found to protect PKM2 from oxidation on Cys 358 by ROS and result in increased apoptosis under high levels of ROS. Treatment of cells with ML285 followed by ROS induction reduces the shunt of glucose through the pentose phosphate pathway and reduces the levels of reduced glutathione available for detoxification.
In vitro activity - Selectivity assay
| Bioassay | ML285 (IC50) |
|---|---|
|
hPyk-M2 |
82 nM |
|
hPyk-M1 (Anti-Target) |
Inactive |
|
hPyk-R (Anti-Target) |
Inactive |
|
hPyk-L (Anti-target) |
Inactive |
Summary /
ML209 is found to have > 100 fold selective against the Pyk-M2 over all 3 isoforms.
Cellular activity - Functional Assay: ROS induced oxidation of C358
Summary /
ML285 protects PKM2 from ROS Induced Oxidation of C358. To determine the affect ML285 had on this inhibitory mechanism, purified recombinant PKM2 is treated with either DMSO or ML285 followed by H2O2 and measured PKM2 activity (Figure 1). This peroxide treatment showed a dose-response inhibition of PKM2 with DMSO control (Figure 1A), but this inhibition was prevented by ML285 treatment (Figure 1B). Extension to a cellular context showed that ML285 activated PKM2 activity in A549 cells in a dose-response manner (Figure 2A). Significant PKM2 activation was seen with ML285 treatment and this activity was slightly enhanced by addition of DTT (Figure 2B). Cells that were diamide treated and followed by addition of activator after cell lysis, did not see as significant of an activation of PKM2 activity (Figure 2C). This shows that ML285 induced a conformation that prevented ROS induced oxidation of C358.
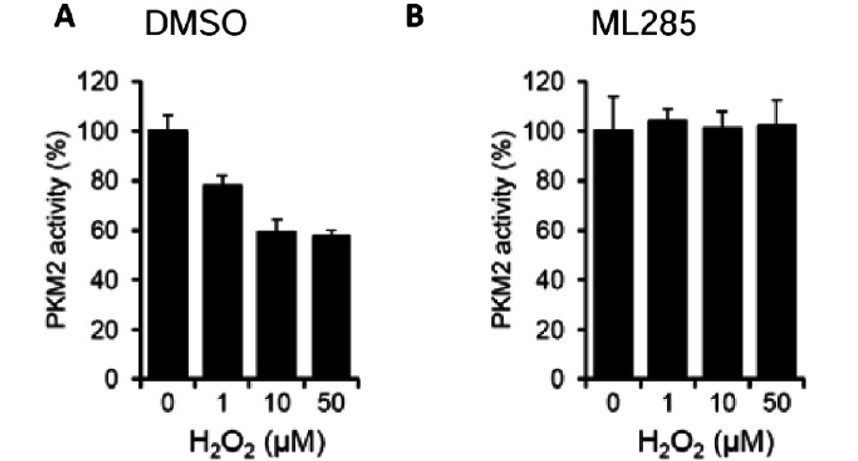
Figure 1. Purified recombinant PKM2 was treated with either A) DMSO or B) 1 μM ML285 followed by H2O2 for 30 minutes, diluted 100-fold and PK activity was assayed.
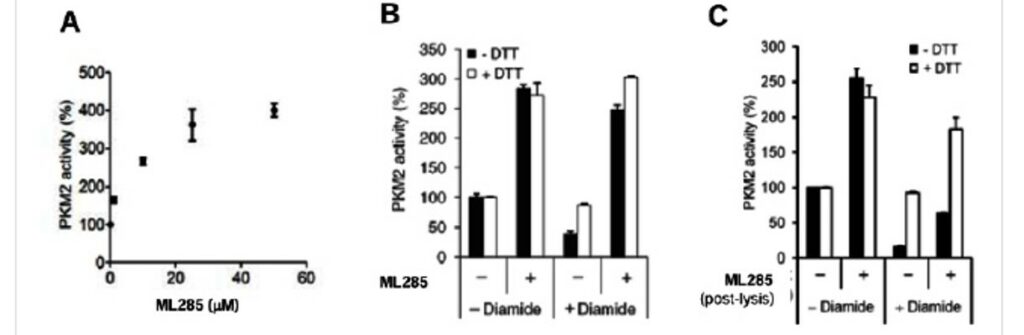
Figure 2. A) ML285 shows a dose-response activation of PKM2 in A549 cells. B) Diamide treatment inhibits PKM2 activity in A549 cells and this inhibition can be prevented by DTT treatment. ML285 activates PKM2 activity in these A549 cells in both diamide treated and untreated cells, and this activation is not affected by DTT treatment. C) ML285 treatment post lysis is not able to significantly prevent diamide induced inhibition of PKM2.
In vitro activity - X-ray crystallography
Summary /
To help elucidate a potential mechanism for ML285’s ability to protect PKM2 from oxidation, we were able to generate a co-crystal structure of ML285 bound to human PKM2 (PDB 3GR4). This structure showed that the PKM2 tetramer contained two equivalents of ML285 each bound at the interface between the A domains of each dimer (Figure 3). The binding site of ML285 was over 30Å away from both the 1,6-fructose bisphosphate and ADP binding pockets. The ML285 binding pocket was lined with equivalent sets of residues provided by each of the PKM2 monomers forming the interface where the activator was accommodated through polar and van der Waals interactions. Two water-mediated hydrogen bonds were observed between the sulfonamide oxygen and the backbone nitrogen of Tyr390. Importantly, Cys358 was located in a β barrel that is not solvent exposed. This suggests that ML285 induction of this tetramer may prevent oxidants from accessing Cys358 and maintain PKM2 in the active conformation.
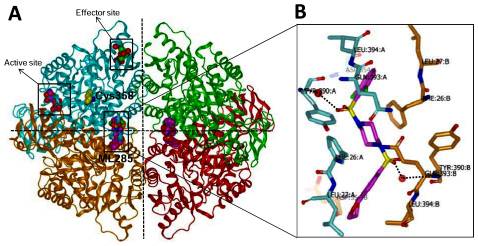
Figure 3. ML285-bound crystal structure of human pyruvate kinase M2 (PDB code: 3GR4) A. Ribbon representation of the human pyruvate kinase M2 tetramer. Each monomer is highlighted in different color. ML285, FBP, ADP and Cys358 are shown in CPK mode. B. Binding orientation of ML285 in dimer-dimer interface. Monomer A is highlighted in cyan, and monomer B is in orange. Water is shown in red sphere. Selected active site amino acid residues are indicated and potential hydrogen-bonds are labeled in black dotted lines.
Cellular activity - Functional assay: Pentose phosphate pathway (PPP)-dependent glucose oxidation to CO2
Summary /
ML285 Reduces Cell’s Ability to Shunt Glucose-6-phosphate to the PPP and Sensitizes Cells to Oxidative Stress. Pyruvate kinase functions at the final stage of glycolysis and is important in controlling flux of glycolytic intermediates. The M2 isoform has been shown to be considerably less active and has been hypothesized to support anabolic processes (Mazurek 2011, Christofk 2008, Vander Heiden 2009, Vander Heiden 2011). In order to understand how ROS induced inhibition of PKM2 may support cancer cell proliferation and/or survival, a study of labeled glucose was undertaken. Cognizant that the pentose phosphate pathway (PPP) is required for reduction of ROS via production of GSH, we analyzed the fate of [1- 14C]glucose. Diamide treatment did increase 14CO2 production via the PPP confirming that shunting through this pathway was activated in response to oxidative stress (Figure 4A). Cells that were treated with ML285 prior to diamide treatment did not show a significant change in 14CO2 derived from [1- 14C]glucose (Figure 4A). Correlatively, the level of glucose-6-phosphate was significantly increased after diamide treatment, but this increase was prevented by treatment with ML285 (Figure 4B). This further signifies that ROS induced oxidation of PKM2 is important for inhibiting glycolysis and providing substrates for the PPP. As previously mentioned, one of the important functions of the PPP shunt under oxidative stress is to produce the NADPH required to provide GSH for ROS detoxification. To this end, GSH levels were measured in a variety of conditions: WT cells, C358S mutant cells, ML285 treated WT cells and PKM1 expressing cells (Figures 4C and D). Levels of GSH were higher in WT cells compared to C358S mutant and these levels observed in WT cells were reduced to levels similar to the mutant upon treatment with ML285 (Figure 4C). GSH concentrations in PKM1 expressing cells were not affected by ML285 treatment, indicating that these compounds are producing this effect by activation of PKM2 and not an off-target effect (Figure 4D).
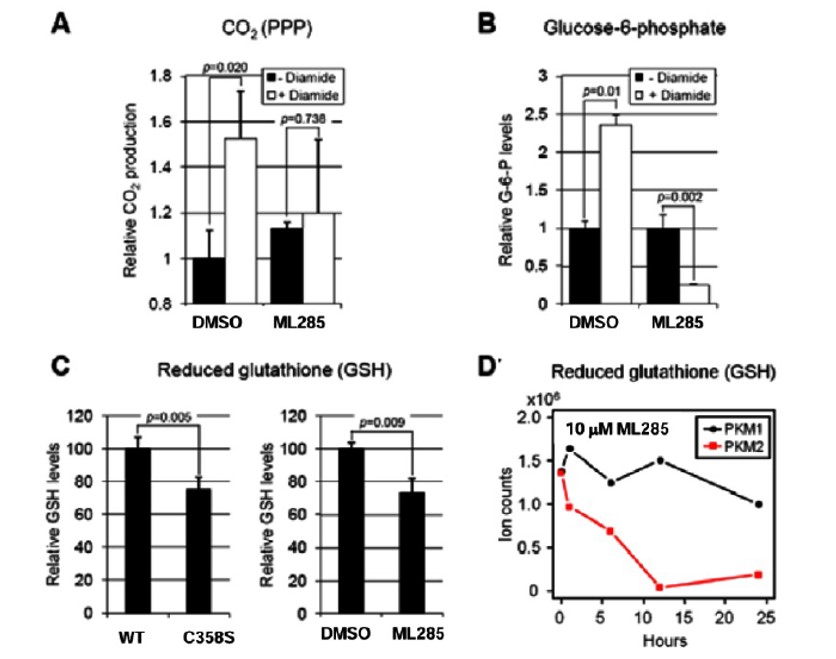
Figure 4. A) [1- 14C] glucose flux through PPP to generate 14CO2 in response to diamide was measured in DMSO and ML285 treated cells. B) Changes in G-6-P levels were measured in response to diamide treatment and were measured in DMSO and ML285 treated cells. C) GSH levels were measured in cells expressing WT PKM2, C358 PKM2 as well as WT PKM2 that was treated with either DMSO or ML285. D) GSH levels measured in A549 cells expressing exclusively either Flag-PKM1 or Flag-PKM2 and treated with ML285. ROS levels were measured with increasing concentrations of H2O2 in either DMSO or ML285 treated cells.
Cellular activity - Functional assay: Survival assay
Summary /
To tie in these activators’ effect on reducing GSH, ROS levels and cell survival were studied. As expected, H2O2 produced higher ROS levels in activator treated cells compared to control and again showed a dose-response with increasing levels of H2O2 (Figure 5A). PKM1 expressing cells showed higher levels of ROS after H2O2 treatement, presumably due to their inability to slow down glycolysis and produce GSH for ROS reduction (Figure 5B). ML285 was able to sensitize cells to oxidant treatment and significant cell death was observed after diamide or H2O2 treatment (Figure 5C). The C358S mutant cells were also more sensitive to diamide treatment, presumably due to an inability to respond to ROS via PKM2 inhibition (Figure 5D).
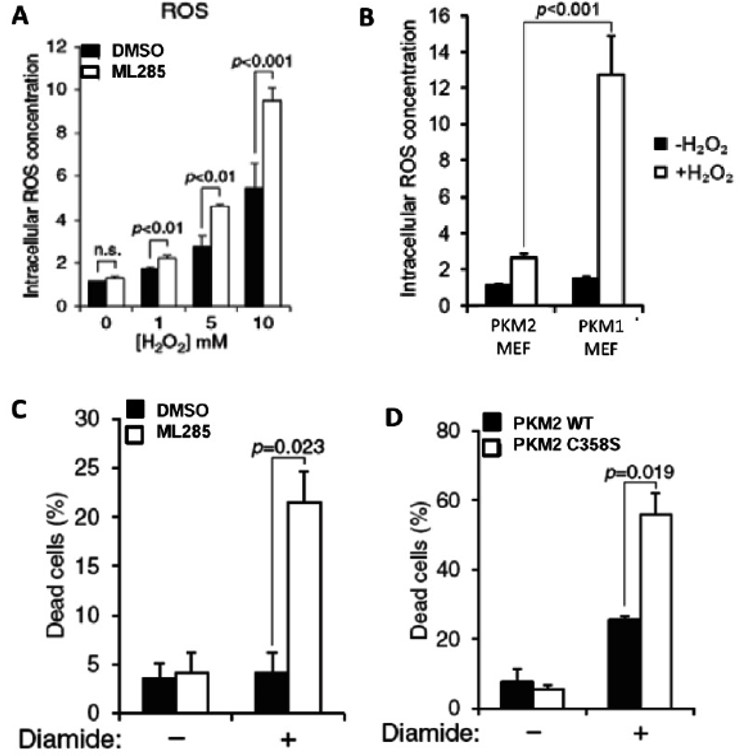
Figure 5. A) Intracellular ROS levels measured with increasing concentrations of H2O2 in either DMSO or ML285 treated A549 cells. B) ROS concentration measured in MEF cell lines expressing either PKM2 or PKM1 with and without H2O2 treatment. C) Cell survival measured in both DMSO and ML285 treated cells with and without diamide treatment. D) Cell survival measure in WT and C358S mutant cells with and without diamide treatment.
References
- Extended Characterization of Activators of Human Muscle isoform 2 Pyruvate Kinase: Summary
- Brimacombe KR, Anastasiou D, Hong BS, et al. ML285 affects reactive oxygen species’ inhibition of pyruvate kinase M2. 2012 Aug 3 [Updated 2013 May 8]. In: Probe Reports from the NIH Molecular Libraries Program [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2010-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK148850/
- RCSB PDB 3GR4
- Mazurek S. Pyruvate kinase type M2: a key regulator of the metabolic budget system in tumor cells. Int J Biochem Cell Biol. 2011;43(7):969-980. doi:10.1016/j.biocel.2010.02.005
- Christofk HR, Vander Heiden MG, Harris MH, et al. The M2 splice isoform of pyruvate kinase is important for cancer metabolism and tumour growth. Nature. 2008;452(7184):230-233. doi:10.1038/nature06734
- Vander Heiden MG. Targeting cancer metabolism: a therapeutic window opens. Nat Rev Drug Discov. 2011;10(9):671-684. Published 2011 Aug 31. doi:10.1038/nrd3504
- Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324(5930):1029-1033. doi:10.1126/science.1160809


