ML267 : Sfp (4'-phosphopantetheinyl transferase Sfp) Inhibitor
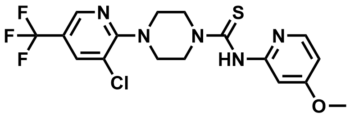
ML267
Target Name
4'-phosphopantetheinyl transferase Sfp
Target Alias
Sfp
Target Class
Transferase
Mechanism of Action
Inhibitor of Sfp
Biological / Disease Relevance
Community-Acquired Methicillin Resistant Staphylococcus aureus Antimicrobial drug, PPTase Biology
In vitro activity
Sfp-PPTase bioassay (IC50)In vitro activity
Gel assay (IC50)Target Information
Since the dawn of the antibiotic era 70 years ago, the evolution of drug resistance has been a persistently evolving threat. This can only be combated by the continued development of new therapies that address resistance mechanisms or engage novel cellular targets. Toward this end, phosphopantetheinyl transferase (PPTase) has been highlighted as a potential target for antibacterial development due to its role in the activation of fatty acid synthase, as well as a myriad of virulence factor-producing machinery. The necessity of this locus to bacterial homeostasis has been confirmed by genetic knockout, and it is intriguing to consider that inhibition of this enzyme may both thwart proliferation and render the bacterium a virulent. However, no chemical matter is currently known that exhibits potent inhibitory activity with this enzyme. To further evaluate the therapeutic potential of PPTase as target for the development of a new class of antibacterial agents, we conducted a quantitative high throughput screening campaign and subsequent medicinal chemistry optimization in pursuit of small molecules that inhibit surfactin PPTase (Sfp). Herein, we detail the discovery of ML267, a probe molecule that has been optimized for nanomolar antagonistic activity with this target. ML267 possesses antibiotic activity against representative Gram positive bacteria, including Community-Acquired Methicillin Resistant Staphylococcus aureus. Moreover, ML267 was profiled for cytoxtoxity (HepG2), acute toxicity (in vivo), and promiscuity against other thiol-sensitive assays (human GST A1-1), and was found to be devoid of activity in these studies. Finally, ML267 possesses a promising in vitro ADME and in vivo PK profile, suggesting its suitability for further testing in animal models of bacterial infection and virulence.
Properties

ML267
NCGC00244182
| Physical & chemical properties | ||||
|---|---|---|---|---|
| Molecular Weight | 431.9 g/mol | |||
| Molecular Formula | C17H17ClF3N5OS | |||
| cLogP | 3.3 | |||
| PSA | 85.6 Ų | |||
| Storage | ||||
| Solubility | ||||
| CAS Number | ||||
SMILES:
COC1=CC(NC(=S)N2CCN(CC2)C3=NC=C(C=C3Cl)C(F)(F)F)=NC=C1
InChI:
1S/C17H17ClF3N5OS/c1-27-12-2-3-22-14(9-12)24-16(28)26-6-4-25(5-7-26)15-13(18)8-11(10-23-15)17(19,20)21/h2-3,8-10H,4-7H2,1H3,(H,22,24,28)
InChIKey:
AIKZMHUYNNXENU-UHFFFAOYSA-N
Activity
Summary activity statement /
ML267 (SID 124398570; CID 53257126) significantly improves the state of the art for the field of PPTase biology, and provides a first in class compound for the Sfp-subtype denotation of this enzyme class. With regard to potency at the directed target, we find that ML267 shows a 5-fold increase over Wyeth Compound 16, displaying an IC50 in the primary screen of 290 nM, with additional coverage of AcpS-PPTase at a potency of 6.93 μM. Furthermore, even greater improvements were seen in MIC experiments, where ML267 a remarkable 38-fold improvement in potency in the common laboratory strain B. subtilis 168, a result that was further enhanced in the genetically modified test strain B. subtilis HM489, whose viability is solely dependent upon an active Sfp gene product. With respect to MIC activity in human pathogens, we observed noteworthy inhibition of Gram positive representative strains of S. aureus, with an 18-fold increase over Wyeth compound 16 in a methicillin resistant strain, with an observed MIC of 3.4 μg/mL. In an act of further attentiveness, this activity was confirmed by an external contract laboratory specializing in anti-infective screening, a result we found particularly motivating. Testing in both Gram negative and fungal pathogens showed that our spectrum of activity was not enveloping with regard to microbial life, a result that is considered acceptable at this stage of an anti-infective discovery campaign (Payne 2007). While these organisms are expected to also depend on PPTase activity for viability, this limited scope of activity may be the result of genetic variability between PPTase loci, the reduced penetrability of the Gram-negative outer membrane (a formidable barrier) (Hancock 1997), or increased efflux capacities in fungal pathogens (Cannon 2009). The in vitro antimicrobial profile of ML267, provides the research community with a unique tool that may be leveraged to 1.) evaluate the role of Sfp-PPTase in bacterial cell viability, 2.) determine the potential of Sfp-PPTase as a drug target in the case of Gram positive bacterial infections, and 3.) assess the role that polyketide, nonribosomal peptide, and fatty acid synthases play in virulence biology. The favorable potency observed against CAMRSA in in vitro antimicrobial studies, coupled to the favorable in vitro and in vivo ADME and PK properties provide a glimmer of hope that a new class of antibiotic compounds acting through novel mechanism of action may soon be within reach.
In vitro activity - Selectivity and Cytotoxicity Assay
| Bioassay | ML267 (IC50) |
|---|---|
|
Sfp-PPTase |
290 nM |
|
HepG2 cells Cytotoxicity (Anti-Target) |
>57 uM |
|
hGST A1-1 (Anti-target) |
Inactive |
Summary /
ML267 is found to have > 196 fold selective against the pathogenic PPTase and showed no toxicity against HepG2 cells at the namomolar concentration. No significant inhibition is observed against the human glutathions S-transferase isoform A1-1 (hGST A1-1).
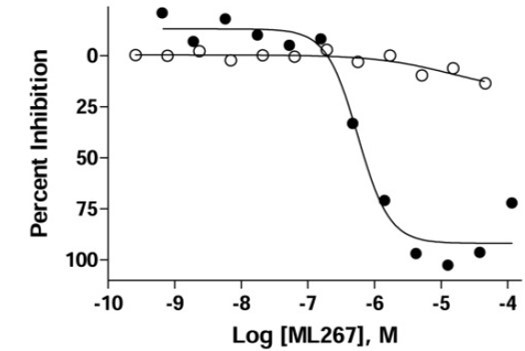
Figure 1. Dose response of Sfp-PPTase activity (●) and the HepG2 cytotoxicity counterscreen (o).
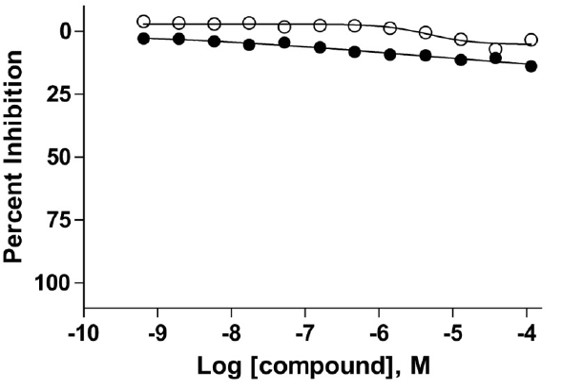
Figure 2. Inhibitory activity of ML267 (●) and 1, CID: 4566836 (o) against the human glutathions S-transferase isoform A1-1 (hGST A1-1).
In vitro and vivo activity - ADME and PK profiling
Summary /
ADME and PK profiling were conducted at Pharmaron Inc. PK profile were conducted using male CD1 mice (6-8 weeks of age). Data was collected in triplicate at 8 time points over a 24 h period.
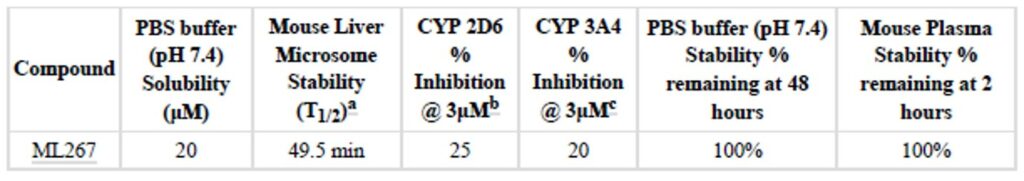
Table 1. ADME profile for ML267. (a) Represents the stability in the presence of NADPH. The probe compound showed no degradation without NADPH present over a 1 hr period. (b) Dextromethorphan was used as the substrate. (c) 6β-hydroxytestosterone was used as the substrate.
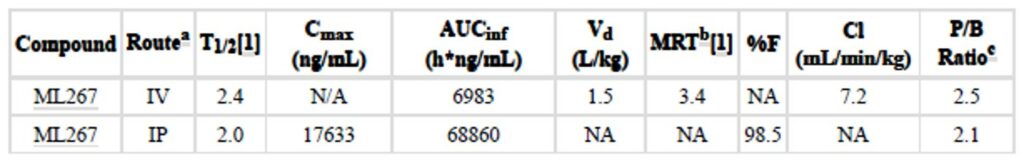
Table 2. In vivo PK (mouse) at 30 mpk IP and 3 mpk IV. (a) Both formulated as a solution (5% DMSO and 10% Solutol in H O). IV: Dosed at 3 mpk; IP: Dosed at 30 mpk. (b) Mean residence time (the time for elimination of 63.2% of the IV dose). (c) Plasma to brain ratio [AUC last (plasma)/AUC last (brain)].
In vitro and vivo activity - Functional assay: Minimum inhibitory concentration assay
Summary /
ML267 significantly improves the state of the art for the field of PPTase biology, and provides a first in class compound for the Sfp-subtype denotation of this enzyme class. With regard to potency at the directed target, we find that ML267 shows a 5-fold increase over Wyeth Compound 16, displaying an IC in the primary screen of 290 nM, with additional coverage of AcpS-PPTase at a potency of 6.93 μM. Furthermore, even greater improvements were seen in MIC experiments, where ML267 a remarkable 38-fold improvement in potency in the common laboratory strain B. subtilis 168, a result that was further enhanced in the genetically modified test strain B. subtilis HM489, whose viability is solely dependent upon an active Sfp gene product. With respect to MIC activity in human pathogens, we observed noteworthy inhibition of Gram positive representative strains of S. aureus, with an 18-fold increase over Wyeth compound 16 in a methicillin resistant strain, with an observed MIC of 3.4 μg/mL. In an act of further attentiveness, this activity was confirmed by an external contract laboratory specializing in anti-infective screening, a result we found particularly motivating. Testing in both Gram negative and fungal pathogens showed that our spectrum of activity was not enveloping with regard to microbial life, a result that is considered acceptable at this stage of an anti-infective discovery campaign (Payne 2007). While these organisms are expected to also depend on PPTase activity for viability, this limited scope of activity may be the result of genetic variability between PPTase loci, the reduced penetrability of the Gram-negative outer membrane (a formidable barrier) (Hancock 1997), or increased efflux capacities in fungal pathogens (Cannon 2009).
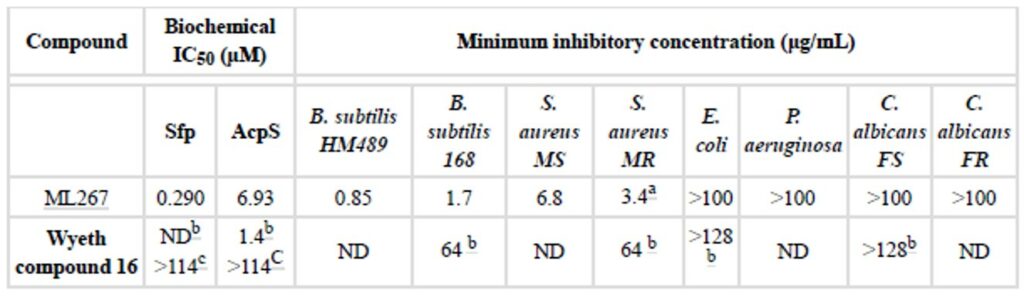
Table 3. Comparison of ML267 to Wyeth Compound 16, an optimized lead described by Wyeth Research. (a) ND. Not determined. Potency confirmed by independent laboratory. NYU Anti-infectives Screening Core, NYU Lagnone Medical Center, NY, NY. (b) Values reported in Joseph-McCarth 2005 paper. (c) Values determined at NCGC.
References
- Probe Development Summary of Inhibitors of Bacillus subtilis Sfp phosphopantetheinyl transferase (PPTase)
- Foley TL, Rai G, Yasgar A, et al. Discovery of ML 267 as a Novel Inhibitor of Pathogenic Sfp phosphopantetheinyl transferase (PPTase) 2012 Mar 21 [Updated 2013 Sep 3]. In: Probe Reports from the NIH Molecular Libraries Program [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2010-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK169448/
- Payne DJ, Gwynn MN, Holmes DJ, Pompliano DL. Drugs for bad bugs: confronting the challenges of antibacterial discovery. Nat Rev Drug Discov. 2007;6(1):29-40. doi:10.1038/nrd2201
- Hancock RE. The bacterial outer membrane as a drug barrier. Trends Microbiol. 1997;5(1):37-42. doi:10.1016/S0966-842X(97)81773-8
- Cannon RD, Lamping E, Holmes AR, et al. Efflux-mediated antifungal drug resistance. Clin Microbiol Rev. 2009;22(2):291-321. doi:10.1128/CMR.00051-08
- Joseph-McCarthy D, Parris K, Huang A, et al. Use of structure-based drug design approaches to obtain novel anthranilic acid acyl carrier protein synthase inhibitors. J Med Chem. 2005;48(25):7960-7969. doi:10.1021/jm050523n


