ML227 : MEN1 (Menin) Inhibitor
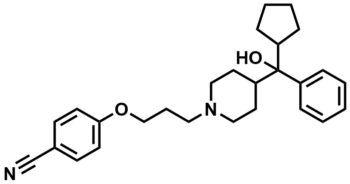
ML227
Target Name
Menin
Target Alias
MEN1
Target Class
Transcription Regulator Protein
Mechanism of Action
Inhibitor of MEN1
Biological / Disease Relevance
Menin-MLL Interaction, Mixed Lineage Leukemia, MLL
In vitro activity
Menin-MLL bioassay (IC50)Cellular activity
MTT cell viabilityTarget Information
A series of lead structures identified from a High Throughput Screen (HTS) targeting the Menin-Mixed Lineage Leukemia (MLL) protein-protein interaction are reported. Two chemical series have been prosecuted to date and one piperidine series was identified to have tractable and rapidly response Structure Activity Relationship (SAR) affording inhibitors of the Menin-MLL interaction with sub-micromolar inhibitory activity. Moreover, preliminary data suggests these compounds display activity in cellular systems relevant to understanding the Menin-MLL pathway and the disease progression. SAR and characterization of the declared probe ML227 from this effort are described.
Properties

ML227
VU0424456
| Physical & chemical properties | ||||
|---|---|---|---|---|
| Molecular Weight | 418.6 g/mol | |||
| Molecular Formula | C27H34N2O2 | |||
| cLogP | 5.2 | |||
| PSA | 56.5 Ų | |||
| Storage | ||||
| Solubility | 6.3 uM in PBS pH 7.4; 40 mM in DMSO | |||
| CAS Number | ||||
SMILES:
N#CC(C=C1)=CC=C1OCCCN(CC2)CCC2C(C3=CC=CC=C3)(O)C4CCCC4
InChI:
1S/C27H34N2O2/c28-21-22-11-13-26(14-12-22)31-20-6-17-29-18-15-25(16-19-29)27(30,24-9-4-5-10-24)23-7-2-1-3-8-23/h1-3,7-8,11-14,24-25,30H,4-6,9-10,15-20H2
InChIKey:
QBITUTDDFZQXDO-UHFFFAOYSA-N
Activity
Summary activity statement /
ML227 (CID 46926632, SID 99432383) is a non-covalent inhibitor of the menin-MLL interaction. ML227 can be accessed synthetically in three-four steps in good overall yield. ML227 shows excellent inhibition in the HTRF assay and demonstrates moderate inhibition on cell proliferation in MLL leukemia cells harboring different translocations of MLL, including: MV4;11 (harboring MLL-AF4 fusion protein), ML-2 (with MLL-AF6 fusion protein) and KOPN-8 (with MLL-ENL fusion protein). ML227 is anticipated to lead to further interest in the development of small molecule inhibitors for the treatment of MLL associated leukemias. ML227 represents the first Menin-Mixed Lineage Leukemia (MLL) small molecule protein-protein interaction inhibitor with sub-micromolar inhibitory activity. This probe will be used by the research community to further elucidate the importance of the menin-MLL interaction in MLL-mediated leukemogenesis and offers a potential drug discovery path to develop small molecules to treat acute lymphoid and myeloid leukemias with MLL rearrangements Translocations of MLL result in acute leukemias with poor prognosis and development of novel therapeutic strategies is highly desired. Leukemogenic activity of MLL fusion proteins is dependent of their interactions with menin, validating the importance of this interaction as a potential drug target for leukemia (Yokoyama 2005, Caslini 2007). Currently, peptide fragments have been identified which are reported to be efficacious in disrupting the Menin-MLL interactions in vitro and in cells (Caslini 2007, Hess 2011). Although development of small peptide disruptors is generally useful for mapping features of the active binding surface and as probes for screening purposes, these tools are typically not viable as lead starting points for small molecule drug discovery. In light of the compelling evidence to date identifying the Menin-MLL fusion protein interaction as a rate limiting interaction necessary for leukemic cell proliferation and blockage of hematopoietic differentiation, the community is earnestly searching for small molecules to disrupt and block the Menin-MLL mediated pathway (Hess 2011). In addition to utilizing a probe molecule to further validate the molecular target, we are focused on identifying good probe compounds to serve as leads for further lead development within the context of the extended probe mechanism. Such compounds might result in novel targeted therapies for MLL-associated acute leukemias, and could also be used a chemical probes to study the biology of MLL and MLL fusion protein mediated leukemogenesis.
Cellular activity - MTT cell viability assay: MLL leukemia cell growth
Summary /
ML227 was tested by MTT cell viability assay for its impact on cell proliferation in MLL leukemia cells harboring different translocations of MLL. Substantial growth inhibition was observed in three different MLL leukemia cells lines: MV4;11 (harboring MLL-AF4 fusion protein), ML-2 (with MLL-AF6 fusion protein) and KOPN-8 (with MLL-ENL fusion protein), with GI50 values at the range of 15-20 uM. In contrast, CID46926611 which shares the same core structure as ML227 but is missing the cyclopentyl group, is a very weak inhibitor of the menin-MLL interaction (IC50 = 234 uM) and shows a very limited effect in MLL leukemia cells. These results demonstrate a strong correlation between the in vitro inhibition of menin-MLL interaction and inhibition of cell growth in MLL leukemia cells for this class of compounds. A broader collection of leukemia cell lines with and without MLL translocations will be tested to further assess specificity and toxicity of ML227.
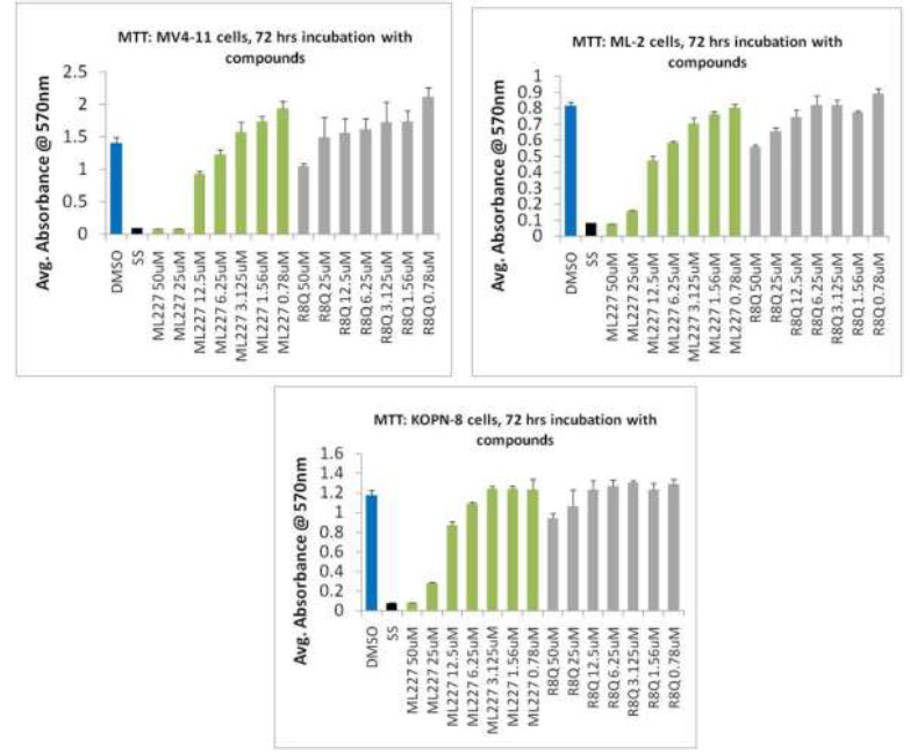
Figure 1. ML227 inhibits MLL leukemia cell growth. Inhibition of cell proliferation in the MLL leukemia cells: MV4;11 (MLL-AF4), ML-2 (MLL-AF6) KOPN-8 (MLL-ENL) induced by ML-227 measured after 72h treatment, as detected by the MTT cell viability assay. Structurally similar CID 46926611 (barcode R8Q), which is a very weak inhibitor of the menin-MLL interaction (IC50 = 234 uM), has a very limited effect on proliferation of MLL leukemia cells. Staurosporin (SS) was used as a positive control in these experiments.
In vitro activity - Mechanism of Action Studies
Summary /
STD NMR studies were used to validate a competitive noncovalent interaction with menin and lead compound MLS001171971 (MIV-1), suggesting a physical binding interaction with the menin protein. Efforts are ongoing to conduct further STD NMR studies using ML227 itself in order to gain a better understanding of the nature of its interaction with menin. Furthermore, the strong correlation between the biochemical and cellular inhibition of cell growth in MLL leukemia cells for ML227 is strongly supportive of this mechanism.
References
- PubChem link: qHTS Assay for Inhibitors Targeting the Menin-MLL Interaction in MLL Related Leukemias: Summary
- Manka J, Daniels RN, Dawson E, et al. Inhibitors of the Menin-Mixed Lineage Leukemia (MLL) Interaction. 2011 Mar 31 [Updated 2013 Feb 28]. In: Probe Reports from the NIH Molecular Libraries Program [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2010-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK133428/
- Yokoyama A, Somervaille TC, Smith KS, Rozenblatt-Rosen O, Meyerson M, Cleary ML. The menin tumor suppressor protein is an essential oncogenic cofactor for MLL-associated leukemogenesis. Cell. 2005;123(2):207-218. doi:10.1016/j.cell.2005.09.025
- Caslini C, Yang Z, El-Osta M, Milne TA, Slany RK, Hess JL. Interaction of MLL amino terminal sequences with menin is required for transformation. Cancer Res. 2007;67(15):7275-7283. doi:10.1158/0008-5472.CAN-06-2369
- Hess, J.; Grembecka, J.; Cierpicki, T.; Compositions and Methods for Treatment of Leukemia. PCT Int. Appl. WO 2011029054, 2011


