ML167 : CLK4 (Dual Specificity CDC-like kinase 4) Inhibitor
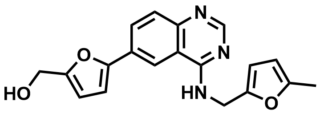
ML167
Target Name
Dual Specificity CDC-like kinase 4
Target Alias
CLK4
Target Class
Serine/ Threonine Protein Kinase
Mechanism of Action
Inhibitor of CLK4
Biological / Disease Relevance
Gene splicing kinase, Splicing modulator
In vitro activity
Clk4 bioassay (IC50)Target Information
Kinases are a major target for pharmacological intervention, and kinase inhibitors (both specific and promiscuous) represent important probes and drugs. Small molecule probes that target specific kinases represent critical tools for exploring and controlling cell function. The Cdc2-like kinases (Clk’s) and the dual-specificity tyrosine phosphorylation-regulated kinases (Dyrk’s) are two classes of enzymes that have been shown to phosphorylate specific proteins within the spliceosome; therefore, they are considered important targets for the modulation of gene splicing events. In addition to the agents reported in these reports, there is only one other reported Clk4 inhibitor, which was found to be somewhat promiscuous versus a kinase panel. Small molecule inhibitors of all 4 isoforms of the Clk family and both the Dyrk1A and Dyrk1B family, with varying selectivity profiles, will be of great utility to the study of these kinases as modulators of gene splicing, as well as other cellular events. The probe described here represents the first fully selective inhibitor of Cdc2-like kinase 4 (Clk4). This probe compound will be useful for the scientific community in unraveling the phenotype associated solely with Clk4 down-regulation without complication arising from the inhibition of related kinases. Particularly, given the reported role of the Clk family as a specific modulator of SR proteins, this probe will be useful in exploring the specific functions of Clk4 in terms of gene splicing.
Properties

ML167
NCGC00188654
| Physical & chemical properties | ||||
|---|---|---|---|---|
| Molecular Weight | 335.4 g/mol | |||
| Molecular Formula | C19H17N3O3 | |||
| cLogP | 2.7 | |||
| PSA | 84.3 Ų | |||
| Storage | ||||
| Solubility | 20.1 uM in PBS, pH 7.4, 23C | |||
| CAS Number | ||||
SMILES:
CC1=CC=C(O1)CNC2=NC=NC3=C2C=C(C4=CC=C(O4)CO)C=C3
InChI:
1S/C19H17N3O3/c1-12-2-4-14(24-12)9-20-19-16-8-13(3-6-17(16)21-11-22-19)18-7-5-15(10-23)25-18/h2-8,11,23H,9-10H2,1H3,(H,20,21,22)
InChIKey:
ROCFOIBAEVAOLQ-UHFFFAOYSA-N
Activity
Summary activity statement /
The Cdc2-like kinases (Clk’s) and the dual-specificity tyrosine phosphorylation-regulated kinases (Dyrk’s) have specified roles in gene splicing. Specifically, the Clk class of enzymes has been shown to phosphorylate the SR proteins, which are a major component of the spliceosome. Dyrk1A has been shown to accumulate in nuclear speckles, where it interacts and activates splicing factors. It has been hypothesized that inhibition of these targets may offer a mechanism to control splicing. This probe represents our continued examination of substituted 6-arylquinazolin-4-amines as Clk/Dyrk inhibitors. Several of the most potent inhibitors, including ML167 (CID 44968231, SID 90944997) were validated as being highly selective within a comprehensive kinome scan. Appropriate aqueous solubility and stability were found for this agent.
In vitro activity - Selectivity
| Bioassay | (IC50) |
|---|---|
|
Clk4 |
136 nM |
|
Clk1 (Anti-Target) |
> 1 uM |
|
Clk2 (Anti-Target) |
> 1 uM |
|
Clk3 (Anti-Target) |
> 1 uM |
|
Dyrk1A (Anti-Target) |
> 1 uM |
|
Dyrk1B (Anti-Target) |
> 1 uM |
Summary /
Kinase Inhibition Profile Study on Inhibitors of CDC-like Kinase 4. ML167 is found to have > 10 fold selective against the Clk4 vs. other Clks and Dyrks.
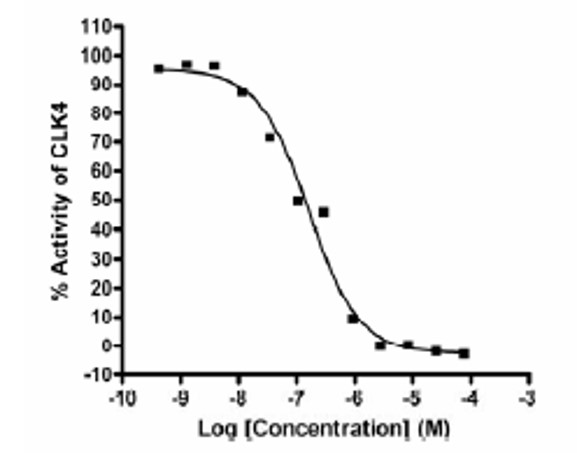
Figure 1. Structure of NCGC00188654 / CID:44968231 / ML167 and dose response curve for in-house Clk4 assay.
In vitro activity - KinomeScan profiling
Summary /
ML167 has been run in Ambit’s KinomeScan profile of 442 kinases. Activities below a 10% threshold are currently being profiled for Kd values versus those targets. We further analyzed ML167 for cell permeability in a commercially available Caco-2 assay. The Papp (A2B) was 32.0 with a percent recovery of 81.4% and the Papp (B2A) was 24.7 with a percent recovery of 81.2%. Atenolol (50% human absorption) and propranolol (90% human absorption) were used as a guide for ranking compounds in terms of permeability. Based on the values for these control compounds, the results ML167 suggest that this agent will be highly cell permeable.
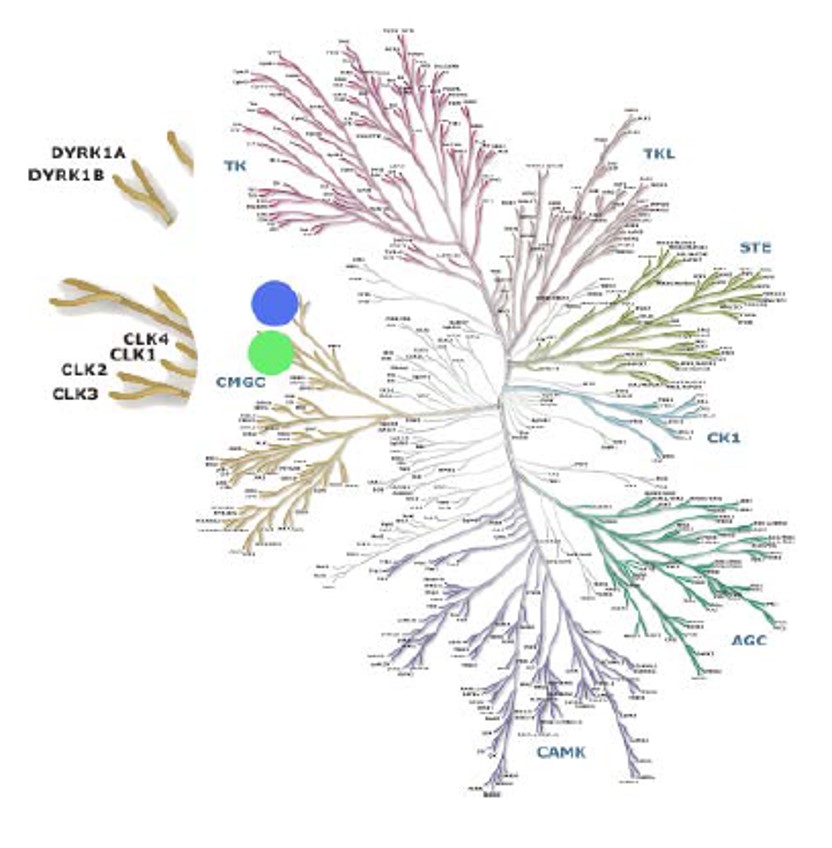
Figure 2. Dendogram representation of the KinomeScan. Results showed two dots (green, blue) representing the only kinases that are inhibited by the probe (Clks and Dyrks).
References
- PubChem link: qHTS for Inhibitors of CDC-like Kinase 4: Summary
- Rosenthal AS, Tanega C, Shen M, et al. An inhibitor of the Cdc2-like kinase 4 (Clk4) 2010 Mar 29 [Updated 2011 Mar 3]. In: Probe Reports from the NIH Molecular Libraries Program [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2010-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK56236/


