ML324 : KDM4E (JMJD2E) (Lysine Demethylase 4E) Inhibitor
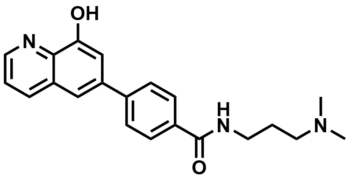
ML324
Target Name
Lysine Demethylase 4E
Target Alias
KDM4E (JMJD2E)
Target Class
Histone Modifying Enzyme
Mechanism of Action
Inhibitor of KDM4E (JMJD2E)
Biological / Disease Relevance
Herpes simplex virus (HSV), Human Cytomegalovirus (hCMV) infection, Epigenetics, Jumonji domain containing proteins (JMJD)
In vitro activity
JMJD2E Bioassay (IC50)Target Information
A critical and dynamic epigenetic post-translational modification involves N-methylation of histone lysine residues by histone methyltransferases. This process was originally thought to be an irreversible epigenetic mark, yet two representative classes of histone lysine demethylases which reverse this process are LSD1/2 and the Jumonji domain containing proteins (JMJD) have emerged. Despite an increased interest in these enzymes as a result of their suspected role in a variety of diseases (e.g. cancer and virus infection), a dearth of potent and cell-permeable inhibitors of the JMJD2 enzymes remain. As such, we sought to discover novel small molecule inhibitors of the JMJD2 family of histone demethylases via a quantitative high throughput screen and subsequent medicinal chemistry optimization campaign. Herein, we describe the discovery and optimization of N-(3-(dimethyamino)propyl-4-(8-hydroxyquinolin-6-yl)benzamide, ML324, a probe molecule that displays submicromolar inhibitory activity toward JMJD2E (in vitro) and possesses excellent in vitro ADME properties. In contrast to previously reported inhibitors of the JMJD proteins, ML324 displays excellent cell permeability providing an opportunity for more extensive cell-based studies of JMJD2 enzymes to be undertaken. In addition, ML324 demonstrates potent anti-viral activity against both herpes simplex virus (HSV) and human cytomegalovirus (hCMV) infection via inhibition viral IE gene expression. Initial siRNA studies revealed that the JMJD2 family of histone demethylases is required for both HSV (herpes simplex virus) and hCMV (human cytomegalovirus) IE gene expression (Liang 2013, Arvin 2007, Fields 2007). Moreover, depletion of JMJD2 suppressed the initiation of infection, blocked the spread of infection to adjacent cells, and resulted in suppression of reactivation in latently infected sensory neurons. Importantly, it was found that depletion of all four proteins (i.e.JMJD2A-D) was required to achieve maximal suppression of IE expression; depletion of individual JMJD2 members only had a modest impact. ML324 suppresses the formation of HSV plaques, even at high MOI, and blocks HSV-1 reactivation in a mouse ganglia explant model of latently infected mice. The studies described herein provide the basis for the use of JMJD2 inhibitors in proof-of-concept animal models for treatment of herpes virus infections and recurrence.
Properties

ML324
NCGC00183808
| Physical & chemical properties | ||||
|---|---|---|---|---|
| Molecular Weight | 349.4 g/mol | |||
| Molecular Formula | C21H23N3O2 | |||
| cLogP | 3.1 | |||
| PSA | 65.5 Ų | |||
| Storage | ||||
| Solubility | ||||
| CAS Number | 1222800-79-4 | |||
SMILES:
CN(CCCNC(C1=CC=C(C2=CC3=CC=CN=C3C(O)=C2)C=C1)=O)C
InChI:
InChI=1S/C21H23N3O2/c1-24(2)12-4-11-23-21(26)16-8-6-15(7-9-16)18-13-17-5-3-10-22-20(17)19(25)14-18/h3,5-10,13-14,25H,4,11-12H2,1-2H3,(H,23,26)
InChIKey:
QDBVSOZTVKXUES-UHFFFAOYSA-N
Activity
Summary activity statement /
ML324 (CID 44143209; SID 85736407) , with its potent inhibition and favorable ADME properties (e.g. solubility, cell permeability, microsomal stability) allows researchers to study the modulation of the JMJD2 family of histone demethylases in vivo. Previous reports have demonstrated the importance of the JMJD2 family of histone demethylases in a variety of important biological processes and diseases (e.g. cancer). In this report, ML324 was used in proof-of-concept studies investigating the role of the JMJD2 proteins in initiation of HSV and hCMV infections and was found to display potent anti-viral activity. Given this promising activity, ML324 could be used in more extensive investigations of the mechanisms involved in chromatin regulation of HSV and other related viruses both in a cellular context and in vivo. Moreover, ML324 could be used to study the role of JMJD2 proteins in the context of cancer biology.
In vitro activity - Selectivity Assay
| ML324 (EC50) | |
|---|---|
|
JMJD2E |
0.92 uM |
|
LSD1 |
Inactive |
|
15-LOX |
Inactive |
|
APE1 |
Inactive |
Summary /
The ML324 chemotype has a general drug-like nature and several marketed drugs possess quinoline scaffolds. In general, 8-hydroxy quinolones are possible metal chelators and could inhibit several metal dependent enzymes. However, these compounds are inactive against several metal dependent enzyme targets, such as LSD1, 15-LOX and APE1. The probe molecule and several analogs obey Lipinski rule and showed excellent ADME properties. The probe does not contain any reactive functional groups or any known structural alerts. In addition, the molecule was stable in PBS buffer, assay buffer, mouse plasma and both in acidic and basic conditions between pH 2-10 over 48 hr. Overall, ML324 is a superior starting point with respect to potency and physiochemical properties for further development.
In vitro and in vivo activity - ADME and PK Profile
| ML324 | |
|---|---|
|
PBS buffer (pH 7.4) Solubility (μM) |
308 |
|
Mouse Liver Microsome Stability (+ NADPH) (T1/2) |
>30 min |
|
Rat Liver Microsome Stability (+ NADPH) (T1/2) |
72 min |
|
Caco-2 (A→B) Papp (10-6 cm/s) |
12.5 |
|
Caco-2 (B→A) Papp (10-6 cm/s) |
45.3 |
|
Efflux Ratio |
3.6 |
|
Plasma Protein Binding (mouse) |
78% |
|
Mouse Plasma Stability (2 h) |
92% |
Summary /
ML324 was found to have an excellent solubility of 308 μM (PBS buffer) which is approaching the upper limit of the detection method. Typically, compounds with such favorable solubility have limited cell permeability as a result of their often polar nature. However, ML324 exhibits good Caco-2 cell permeability with a Papp of 12.5 (10-6 cm/s), albeit fairly significant efflux was observed, suggesting that it may be a Pgp substrate. These findings will need to be further explored by conducting the experiment in the presence of a Pgp inhibitor and see if the efflux is attenuated. We will also test permeability using the MDR1-MDCKII cell line, which also expresses Pgp to confirm the result. Importantly, ML324 possessed excellent microsomal stability in the presence of both mouse and rat liver microsomes with a T1/2 of >30 minutes and 72 minutes, respectively. Moreover, ML324 exhibited stability in plasma and aqueous solutions at various pH (see above) with low plasma protein binding of 78%. These data suggests that ML324 will have suitable pharmacokinetics/exposure for use in proof of concept animal models for HSV infection or studies requiring a JMJD2 inhibitor.
This study was conducted by Pharmaron Inc. and was determined by LC/MS/MS. The probe compound showed no degradation without NADPH present over a 1 hr period.
Cellular activity - Viral immediate early (IE) gene expression assay
Summary /
The initial study tested the ability of ML324 to inhibit HSV viral IE infection in HFF cells (Hamada 2009). ML324, potently reduced IE gene expression (IC50 = 10 μM) compared to 0.75 mM for DMOG, without an impact on the expression of the cellular controls Sp1, S15 and TBP. Next, HFF cells were treated with various concentrations of ML324 and infected with HSV-1 (0.1 pfu/cell) for 24 hr. ML324 reduced viral yields in a dose-dependent manner (~4-5 logs at 25 μM, Figure 1B) whereas 1.5 mM of DMOG was required to achieve the same reduction (data not shown). HSV-1 infected MRC-5 cells were treated with DMSO or ML324 at various time points (2 hr pre-infection to 4 hr post infection) and mRNA levels of IE viral genes (ICP4, ICP22, ICP27) were measured relative to DMSO at 4 hr post infection. As shown in Figure 12A, ML324, markedly reduced the gene transcription of all 4 viral IE genes, even when added 2 hr post-infection (>50% reduction). ML324 was tested for its ability to reduce viral yields in MRC-5 cells infected at high multiplicity of infection (MOI) (Figure 2D), and was found to display comparable viral yield reduction at half the concentration needed for ACV (acyclovir), 50 μM vs. 100 μM respectively. Furthermore, ML324 is shown as an efficacious antiviral agent, by observing significant reduction in HSV plaque formation in Vero cells and MRC-5 with 50 μM, as compared to 100 μM ACV (Figure 2B-C).
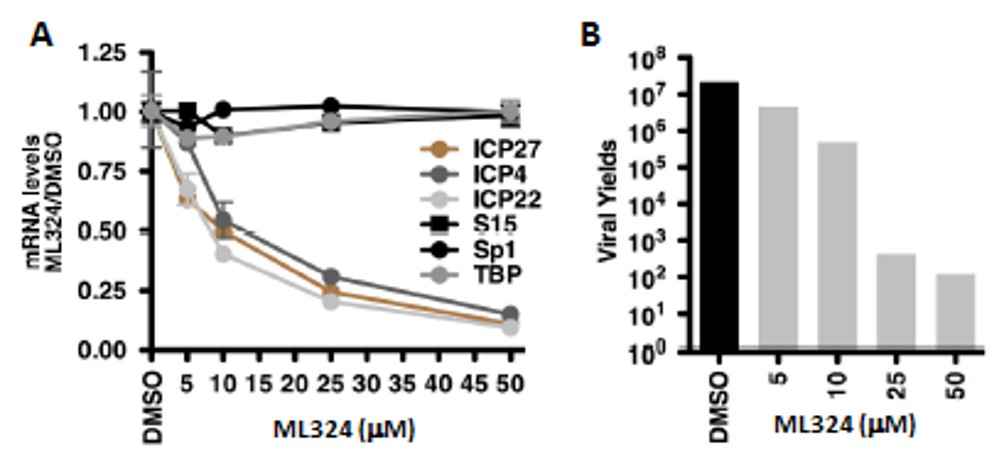
Figure 1. (A) Viral IE (ICP4, ICP27, ICP22) and control (S15, Sp1, TBP) mRNA levels in HFF cells treated with DMSO or the indicated concentrations of ML324 for 3 hrs and infected with HSV-1 (0.1 pfu/cell) for 3 hr. (B) Viral yields from HFF cells treated with DMSO or ML324 for 3 hr and infected with HSV-1 (0.1 pfu/cell) for 24 hr in the presence of the drugs.
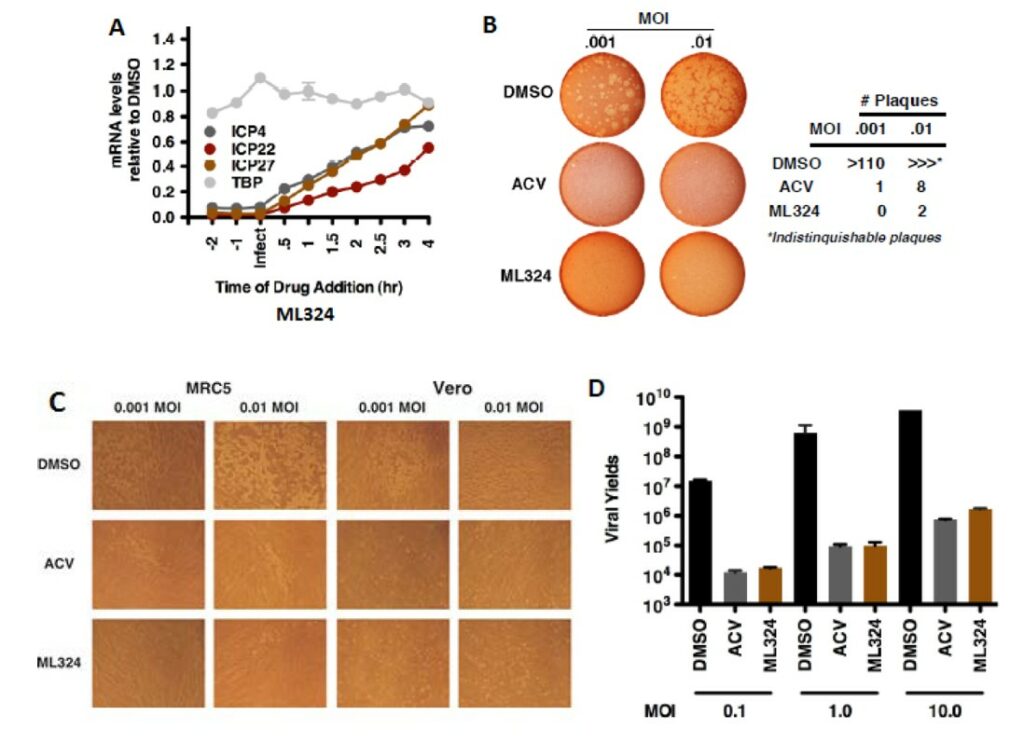
Figure 2. (A) MRC-5 cells were treated with DMSO or ML324 at the indicated time relative to infection with HSV-1 (1.0 MOI). Viral and cellular mRNA levels were determined at 4 hr post infection. (B) Plaque assay of Vero cells infected with HSV-1 at the indicated MOI for 12 hr, followed by the addition of DMSO, ACV (100 μM), or ML324 (50 μM) for 48 hr. (C) Representative fields of MRC-5 and Vero cells infected with HSV-1 for 12 hr followed by addition of DMSO, ACV (100 μM) or ML324 (50 μM) for 48 hr (D) MRC-5 cells treated with DMSO or 50 μM ML324 were infected with HSV-1 at the indicated MOI. Viral IE and cellular mRNA levels were quantitated at 2 hr post infection; viral yields were determined at 24 hr post infection.
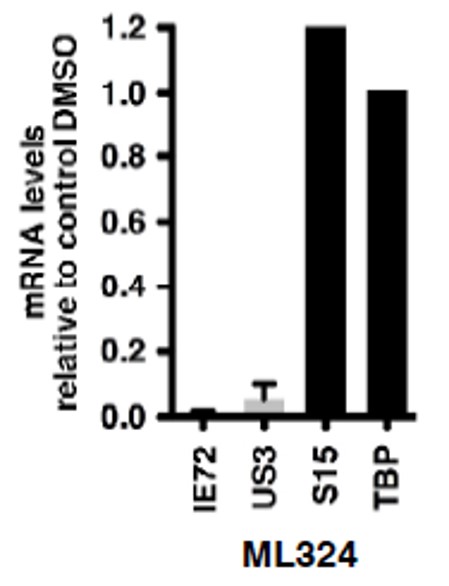
Figure 3. MRC-5 cells were treated with DMSO or 50 mM ML324 for 3 hr, followed by infection with hCMV (0.1 pfu/cell) for 5 hr. mRNA levels of viral and cellular controls are expressed relative to cells treated with DMSO. Data shown are means +/- SEM.
Cellular activity - HSV infection assay
Summary /
To investigate the effect of ML324 on the spread of infection to adjacent cells, MRC-5 cells were infected with HSV-1 and visualized by immunofluorescent staining for UL29 (HSV DNA replication protein) (Figure 4). ML324 blocked viral gene expression at an early stage prior to expression of the viral replication protein UL29, thereby reducing the number of cells exhibiting viral antigens.
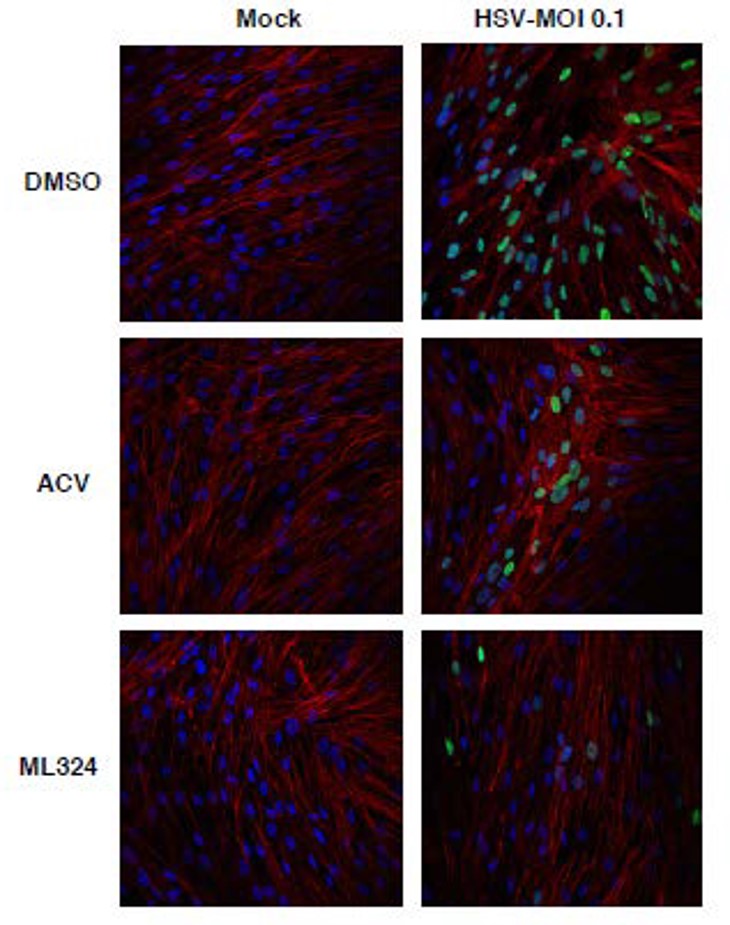
Figure 4. Immunofluorescent staining for the HSV DNA replication protein UL29. MRC-5 cells were infected with HSV-1 (0.1 MOI) for 8 hr, followed by the addition of DMSO, ACV (100 μM), or ML324 (50 μM) for 12 hr. Cells were stained with anti-UL29 (green), Dapi (blue), and Phalloidin-647 (F-actin, red).
Cellular activity - Mouse sensory ganglia HSV-1 infection assay
Summary /
ML324 exhibited significant antiviral activity in the mouse sensory ganglia explant model of HSV reactivation. First, ML324 reduced the viral yield per ganglia by 4.5 logs at 50 μM compared to DMOG which required concentrations up to 2 mM to achieve 1.5 log reduction (Figure 5A). Immunofluorescent staining of explanted ganglia sections (Figure 5B & D) revealed that ML324 was capable of suppressing the primary viral reactivation events. The spread of the reactivated viral infection of the ganglia was demonstrated in the DMSO control where the viral lytic gene (UL29) expression was observed in both isolated and clustered neurons (Figure 5D). However, upon treatment with ML324, significant reduction in both isolated and clustered UL29(+) neurons was observed (note: ML324 was more efficacious than ACV in single neurons). Importantly, robust viral replication was observed following ML324 withdrawal which indicates the reduction in reactivation by ML324 was not simply due to the inability of the ganglia to support viral replication (Figure 5C). In addition to the encouraging results obtained for the herpes simplex virus (HSV) reported herein, ML324 also demonstrated comparable activity towards β-herpes human Cytomegalovirus (hCMV) which causes mortality in immuno compromised patients and is the most significant cause for viral birth defects (Arvin 2007, Fields 2007). These data support the notion that the JMJD2 demethylases are required for both HSV and hCMV IE gene expression and demonstrate the effectiveness of ML324 as an antiviral agent.
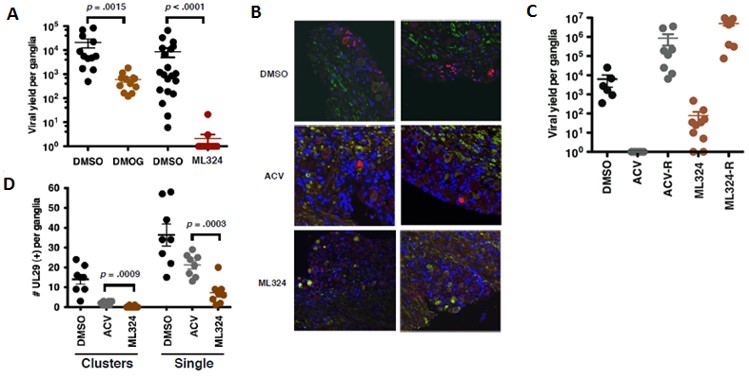
Figure 5. (A) Viral yields from HSV-1 latently infected trigeminal ganglia explanted in the presence of DMSO, or 2 mM DMOG or 50 μM ML324 for 48 hr. [DMSO vs DMOG; Wilcoxon paired 2-tailed t test, p = .0015, n = 12; DMSO vs ML324, p < .0001, n = 20]. (B & D) Latently infected trigeminal ganglia were explanted in the presence of DMSO, 100 μM ACV, or 50 μM ML324 for 48 hr. Sections were costained with anti-UL29 (red), neurofilament 200 (green), and DAPI (blue) and scored for UL29(+) cell clusters (Clusters) and individual neurons (Single). [ACV vs ML324; 2-tailed t test, n = 48 sections representing 8 ganglia). The total number of UL29(+) per ganglia are graphed (D) and representative sections illustrated (B). Data shown are means +/- SEM. (C) Latently infected ganglia were explanted in the presence of DMSO, ACV (100 μM), or ML324 (50 μM) for 48 hr. Viral yields were determined from one half of each ganglia at 48 hr (DMSO, ACV, ML324) and from the other half of each ganglia after drug reversal for an additional 72 hr (ACV-R, ML324-R).
References
- Probe Summary for Inhibitors of Human Jumonji Domain Containing 2E (JMJD2E)
- Rai G, Kawamura A, Tumber A, et al. Discovery of ML324, a JMJD2 demethylase inhibitor with demonstrated antiviral activity. In: Probe Reports from the NIH Molecular Libraries Program. Bethesda (MD): National Center for Biotechnology Information (US); December 17, 2012
- Liang Y, Vogel JL, Arbuckle JH, et al. Targeting the JMJD2 histone demethylases to epigenetically control herpesvirus infection and reactivation from latency. Sci Transl Med. 2013;5(167):167ra5. doi:10.1126/scitranslmed.3005145
- Arvin A, Campadelli-Fiume G, Mocarski E, et al., eds. Human Herpesviruses: Biology, Therapy, and Immunoprophylaxis. Cambridge: Cambridge University Press; 2007.
- Fields, B. N., Knipe, D. M., & Howley, P. M. (2007). Fields virology. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins. vol. 2, pp. 2701-2772
- Hamada S, Kim TD, Suzuki T, et al. Synthesis and activity of N-oxalylglycine and its derivatives as Jumonji C-domain-containing histone lysine demethylase inhibitors. Bioorg Med Chem Lett. 2009;19(10):2852-2855. doi:10.1016/j.bmcl.2009.03.098


