ML165 : ITA2B (ITGA2B) (Platelet Integrin alphallb-beta3) Inhibitor
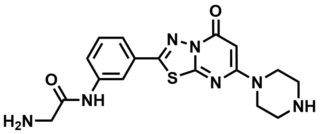
ML165
Target Name
Platelet Integrin alphallb-beta3
Target Alias
ITA2B (ITGA2B)
Target Class
Integrin
Mechanism of Action
Inhibitor of ITA2B (ITGA2B)
Biological / Disease Relevance
Glanzmann Thrombasthenia; Thrombosis; Hemostasis
Cellular activity
αIIbβ3 receptor Platelet Adhesion (IC50)Cellular activity
Platelet Aggregation (IC50)Target Information
The αIIbβ3 receptor plays a vital role in both hemostasis and thrombosis, with deficiency of the receptor leading to Glanzmann thrombasthenia, and uncontrolled activation of the receptor producing thrombosis and blood vessel occlusion in animal models and humans (Ginsberg 2005, Shattil 2004, Seligsohn 2002). Current inhibitors of this key integrin receptor include a monoclonal antibody fragment and several RGD peptide mimetics (Coller 1997, Hartzman 1992). Use of these agents can be problematic, as they engage the β3 subunit MIDAS metal ion and are capable of priming the receptor into an artificial activation conformation. This probe does not bind to the β3 subunit MIDAS metal ion as judged by molecular dynamic simulation, and will be useful for studying selective inhibition of the αIIbβ3 receptor.
Properties

ML165
RUC-2
| Physical & chemical properties | ||||
|---|---|---|---|---|
| Molecular Weight | 385.1321 g/mol | |||
| Molecular Formula | C17H19N7O2S | |||
| cLogP | -0.2 | |||
| PSA | 141 Ų | |||
| Storage | ||||
| Solubility | 10 mM in DMSO | |||
| CAS Number | ||||
SMILES:
NCC(NC1=CC(C2=NN3C(S2)=NC(N4CCNCC4)=CC3=O)=CC=C1)=O
InChI:
1S/C17H19N7O2S/c18-10-14(25)20-12-3-1-2-11(8-12)16-22-24-15(26)9-13(21-17(24)27-16)23-6-4-19-5-7-23/h1-3,8-9,19H,4-7,10,18H2,(H,20,25)
InChIKey:
ITNCYPYTFKVCFI-UHFFFAOYSA-N
Activity
Summary activity statement /
Continued examination of substituted 5H-[1,3,4]thiadiazolo[3,2-a]pyrimidin-5-ones as inhibitors of the platelet αIIbβ3 receptor, resulted in the optimized agent ML165 (NCGC00183896, CID 44820665, SID 89449681) his agent represents the most potent non-RGC mimetic inhibitor of the αIIbβ3 receptor, and due to its unique biding mechanism, offers a novel tool to study this receptor. Appropriate aqueous solubility and stability was found for this agent.
In vitro activity - Selectivity and Cytotoxicity Assay
| Bioassay | (IC50) |
|---|---|
|
αIIbβ3 Receptor Platelet Adhesion |
1.100 uM |
|
αVβ3 Receptor (Anti-Target) |
>100 uM |
|
Platelet Aggregation |
163 nM |
Summary /
ML165 is found to have > 100 fold selective for αIIbβ3 compared to αVβ3 and does not induce the β3 LIBS epitope. In contrast to prior art tirofiban and eptifibatide, neither ML165 (nor prior art RUC-1) induced recruitment of IgG in 10 of 12 patient cell lines with eptifibatide-dependent thrombocytopenia. ML165 is also found to be more potent than prior art RUC-1 and possesses a unique binding mode to human αIIbβ3 receptor.
In vitro activity - Mechanism of Action Studies
Summary /
Studies into the structural basis for the binding of ML165 to human αIIbβ3 have revealed a novel mechanism of action. ML165 is an orthosteric inhibitor.
References
- PubChem link: Inhibitors of Platelet Integrin alphallb-beta3: Summary
- McCoy J, Shen M, Huang W, et al. Inhibitors of Platelet Integrin αllbβ3. 2010 Mar 27 [Updated 2011 Mar 3]. In: Probe Reports from the NIH Molecular Libraries Program [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2010-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK56230/
- Ginsberg MH, Partridge A, Shattil SJ. Integrin regulation. Curr Opin Cell Biol. 2005 Oct;17(5):509-16. doi: 10.1016/j.ceb.2005.08.010. PMID: 16099636
- Shattil SJ, Newman PJ. Integrins: dynamic scaffolds for adhesion and signaling in platelets. Blood. 2004 Sep 15;104(6):1606-15. doi: 10.1182/blood-2004-04-1257. Epub 2004 Jun 17. PMID: 15205259.
- Seligsohn U. Glanzmann thrombasthenia: a model disease which paved the way to powerful therapeutic agents. Pathophysiol Haemost Thromb. 2002 Sep-Dec;32(5-6):216-7. doi: 10.1159/000073569. PMID: 13679645
- Coller BS. Platelet GPIIb/IIIa antagonists: the first anti-integrin receptor therapeutics. J Clin Invest. 1997;99(7):1467-1471. doi:10.1172/JCI119307
- Hartman GD, Egbertson MS, Halczenko W, Laswell WL, Duggan ME, Smith RL, Naylor AM, Manno PD, Lynch RJ, Zhang G, et al. Non-peptide fibrinogen receptor antagonists. 1. Discovery and design of exosite inhibitors. J Med Chem. 1992 Nov 27;35(24):4640-2. doi: 10.1021/jm00102a020. PMID: 1469694.


