ML155 : GBA (Glucocerebrosidase (N370S mutant)) Modulator

ML155
Target Name
Glucocerebrosidase (N370S mutant)
Target Alias
GBA
Target Class
Hydrolase
Mechanism of Action
Modulator of GBA
Biological / Disease Relevance
Gaucher; ER-lysosomal trafficking; chaperone of glucocerobrosidase
In vitro activity
N370S GC bioassay (IC50)Cellular activity
Chaperone activity assay (IC50)Target Information
The probe is able to inhibit the hydrolytic activity of the N370S mutant form of glucocerebrosidase, as well as wild type glucocerebrosidase, in tissue homogenate assays. The probe does not inhibit purified glucocerebrosidase, but the cellular activity of the enzyme is known to be dependent on interactions with other factors, such as Saposin C. Importantly, the probe increased glucocerebrosidase translocation to the lysosome in Gaucher patient-derived fibroblasts homozygous for the N370S mutation, and can be used to study ER-lysosomal trafficking of clinically relevant GC mutants in vitro. This probe may be a useful lead for the clinical development of a chemical chaperone of glucocerebrosidase.
Properties

ML155
NCGC00182292
| Physical & chemical properties | ||||
|---|---|---|---|---|
| Molecular Weight | 486.6 g/mol | |||
| Molecular Formula | C26H22N4O2S2 | |||
| cLogP | 5.2 | |||
| PSA | 103 Ų | |||
| Storage | ||||
| Solubility | ||||
| CAS Number | 10 mM in DMSO | |||
SMILES:
O=S(N1CCN(C2=NC(C3=CC=CS3)=NC4=C2C=CC=C4)CC1)(C5=CC6=CC=CC=C6C=C5)=O
InChI:
1S/C26H22N4O2S2/c31-34(32,21-12-11-19-6-1-2-7-20(19)18-21)30-15-13-29(14-16-30)26-22-8-3-4-9-23(22)27-25(28-26)24-10-5-17-33-24/h1-12,17-18H,13-16H2
InChIKey:
UREJDUPKGMFJRU-UHFFFAOYSA-N
Activity
Summary activity statement /
ML155 (CID 40225210, SID 85267237) is described as a probe that is able to inhibit the hydrolytic activity of the N370S mutant form of glucocerebrosidase, as well as wild type glucocerebrosidase, in tissue homogenate assays. The probe does not inhibit purified glucocerebrosidase, but the cellular activity of the enzyme is known to be dependent on interactions with other factors, such as Saposin C. Importantly, the probe increased glucocerebrosidase translocation to the lysosome in Gaucher patient-derived fibroblasts homozygous for the N370S mutation, and can be used to study ER-lysosomal trafficking of clinically relevant GC mutants in vitro. This probe may be a useful lead for the clinical development of a chemical chaperone of glucocerebrosidase.
In vitro activity - Selectivity
| Bioassay | ML156 (IC50) |
|---|---|
|
N370S GC |
0.330 uM |
|
Chaperone activity (N370S GC fibroblast) |
0.50 uM |
|
Alpha-glucosidase (Anti-Target) |
> 57 uM |
|
Alpha-galactosidase (Anti-Target) |
> 57 uM |
Summary /
ML155 is found to have > 100 fold selective against glucocerebrosidase. Selectivity was measured against other two lysosomal hydrolases: alpha glucosidase (AID 2578) and alpha galactosidase (AID 2577). All tested compounds from this quinazoline series show selectivity for inhibiting glucocerebrosidase. None of them show inhibitory activity towards alpha glucosidase or alpha galactosidase at concentrations up to 57μM.
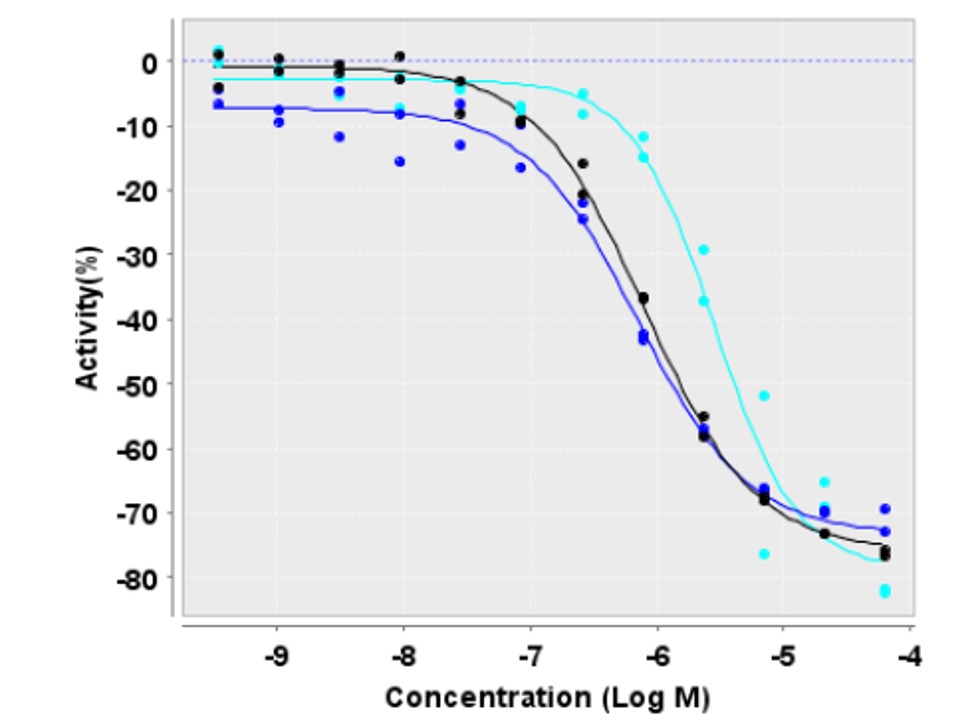
Figure 1: Inhibitory curves of ML155 / compound 13 (CID 40225210), against spleen homogenate homozygous for N370S GC using 4-methylumbelliferyl-beta-D-glucopyranoside (curve in black) and resorufin beta-D-glucopyranoside as substrates (curve in blue), and wildtype spleen homogenate using 4 methylumbelliferyl-beta-D glucopyranoside (curve in cyan; AID 2592).
Cellular activity - Chaperone assay
Summary /
To demonstrate chaperone activity, the capacity of ML156 and ML155 to increase the translocation of GC to the lysosome was measured (8, 16, 30, 31). In this experiment, wildtype and mutant fibroblasts were incubated for five days with compound 8i, followed by cell fixation and staining with a selective fluorescent GC antibody. Compounds able to promote trafficking from the ER to the lysosome increased the fluorescent lysosomal signal. DMSO and isofagomine were used as negative and positive controls. Figure 2 shows the increment of signal in both cell lines that resulted from treatment, confirming the chaperone capacity of these compounds.
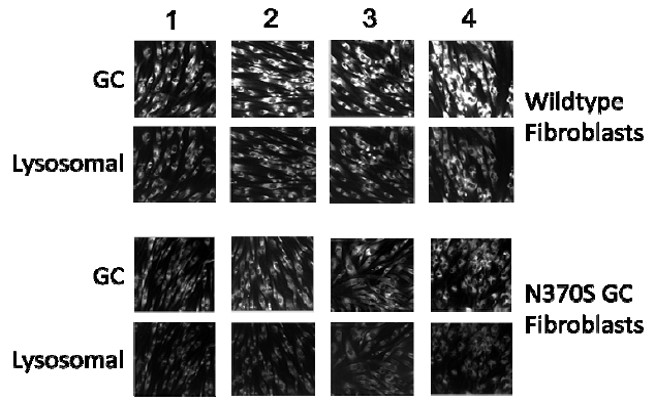
Figure 2: Chaperone activity of compound 13, NCGC00182292 (CID 40225210), and others using wildtype and homozygous, mutant N370S GC fibroblasts. Two genotypes of fibroblasts, fibroblasts homozygous for wildtype GC (top) and fibroblasts homozygous for N370S GC (bottom) were stained both with a Cy3-labeled antibody for GC protein content (first row) and a FITC-labeled antibody specific for lysosomal compartments (LAMP1; second row) after treatment with (1) DMSO vehicle, (2) 10μM Isofagomine (CID 447607), (3) 10μM compound 13, NCGC00182292 (CID 40225210), and (4) 1μM NCGC00159568 (CID 9893924). Several compounds, including isofagomine (CID 447607) and compound 13, NCGC00182292 (CID 40225210), show increased lysosomal GC protein after treatment.
In vitro activity - Mechanism of Action
Summary /
In enzyme kinetics assays, the probe, along with other glucocerebrosidase inhibitors such as isofagomine demonstrated mixed mode inhibition – neither competitive nor non-competitive enzyme kinetics. It is obvious, from x-ray crystal structure studies of isofagomine binding, that the compound binds in the active site. Thus, the current homogenate-based assays might be ill-suited for such kinetic characterization.
References
- PubChem link: qHTS Assay for Inhibitors and Activators of N370S glucocerebrosidase as a Potential Chaperone Treatment of Gaucher Disease: Summary
- Motabar O, Huang W, Marugan JJ, et al. Identification of Modulators of the N370S Mutant Form of Glucocerebrosidase as a Potential Therapy for Gaucher Disease - Chemotype 2. 2010 Mar 24 [Updated 2011 Mar 25]. In: Probe Reports from the NIH Molecular Libraries Program [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2010-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK56229/
- Lieberman RL, Wustman BA, Huertas P, Powe AC Jr, Pine CW, Khanna R, Schlossmacher MG, Ringe D, Petsko GA. Structure of acid beta-glucosidase with pharmacological chaperone provides insight into Gaucher disease. Nat Chem Biol. 2007 Feb;3(2):101-7. doi: 10.1038/nchembio850. Epub 2006 Dec 24. PMID: 17187079


