ML068 : HBB (Beta-Globin) Modulator
ML068
Target Name
Beta-Globin
Target Alias
HBB
Target Class
Transport Protein
Mechanism of Action
Modulator of HBB
Biological / Disease Relevance
Thalassemia, Cooley's anemia, Splicing modulators
In vitro activity
B-globin splicing (Exon 1) assay AC50Target Information
β-thalassemia, also known as Cooley’s anemia, is a genetic blood disorder caused by a genetic mutation at the β-globin locus. Although over 200 mutations have been revealed to cause β-thalassemia, about 10 mutations are responsible for over 90% of the cases worldwide (Schwartz 1995); these include mutations which adversely affect the splicing pattern of the β-globin gene. A mutation in the second intron of β-globin at position 654 (called IVS2-654) leads to the creation of an aberrant 5’ splice donor site and activation of a 3’ cryptic splice acceptor site (Sazani 2003). This IVS2-654 mutation is frequently found in patients from Thailand and China (Sierakowska 1996). This form of altered splicing leads to the inclusion of a portion of intron 2 in the mature spliced mRNA, and ultimately results in a prematurely terminated protein. Perturbation of β-globin synthesis affects the ability of red blood cells to carry oxygen, and the severity of the disease depends on the nature of the mutation. Thalassemia is one of the most common genetic disorders worldwide, and β-thalassemia affects a large number of people in the Middle East, South East Asia, Mediterranean basin, and Africa (Rund 2005).
Currently, there is no effective drug treatment for β-thalassemia. The ability to target and control pre-mRNA splicing would be an important breakthrough in the search for therapeutics for such diseases. It has been found that splice switching oligonucleotides can manipulate splicing by hybridizing the pre-mRNA at elements critical for splicing factor recognition. This program was designed to use a cell line containing a mutant intron-2 of IVS2-654 inserted into a stably expressed EGFP gene for identifying the small molecule modulators that block the aberrant splicing.
Project Team
Properties

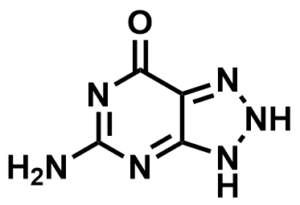
ML068
8-Azaguanine
| Physical & chemical properties | ||||
|---|---|---|---|---|
| Molecular Weight | 152.11 g/mol | |||
| Molecular Formula | C4H4N6O | |||
| cLogP | -0.71 | |||
| PSA | 109 | |||
| Storage | -20 | |||
| Solubility | Insoluble | |||
| CAS Number | ||||
SMILES:
NC(N=C1NNN=C12)=NC2=O
InChI:
InChI=1S/C4H4N6O/c5-4-6-2-1(3(11)7-4)8-10-9-2/h(H4,5,6,7,8,9,10,11)
InChIKey:
LPXQRXLUHJKZIE-UHFFFAOYSA-N
Activity
Summary activity statement /
ML068 (SID11112293, CID135403646, 8-Azaguanine) is a purine analogue with potential antineoplastic activity. 8-Azaguanine interferes with the modification of transfer ribonucleic acid (tRNA) by competing with guanine for incorporation into tRNA catalyzed by the enzyme tRNA-guanine ribosyltransferase (tRNA-guanine transglycosylase). Altered guanine modification of tRNA has been implicated in cellular differentiation and neoplastic transformation. 8-Azaguanine also inhibits the formation of 43S and 80S initiation complexes, thereby interfering with initiation of translation and inhibiting protein synthesis. This agent inhibits tumor cell growth and stimulates cell differentiation.
In vitro activity - EGFP-ISV2-654 splicing assay
Summary /
ML068 was confirmed to correct the aberrant splicing in the EGFP-IVS2-654 cell line in a concentration dependent manner, with an EC50 of 3.3 uM (Fig. 4).
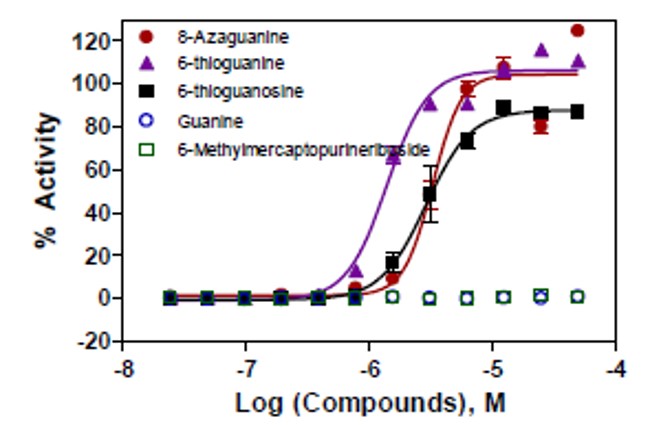
Figure 4. Concentration responses on correction of aberrant splicing caused by the ISV2-654 mutation by six purine analogs. The AC50 values of 8-azaguanine (ML068), thioguanine, thioguanosine and 6-Mercaptopurine riboside were 3.3, 1.2, 2.0 and 2.0 μM, respectively. The other two purine analogs, Quanine and 6-Methylmercaptopurine riboside, were not active in this assay.
In vitro activity - RT-PCR Assay
Summary /
RT-PCR assay. A RT-PCR assay was used to measure the ability of these confirmed compounds to correct the aberrant splicing due to the intron-2 of IVS2-654 mutation. We found that its activity on correction of aberrant splicing was confirmed by quantifying relative expression of the correctly- and aberrantly-spliced mRNAs with a RT-PCR experiment (Fig. 5a). Furthermore, in another β-globin-IVS2 cell line that contained the full-length mutant β-globin gene, 8-azaguanine was able to correct the aberrant splicing of the thalassemic β-globin transcript as measured by the RT-PCR method (Fig. 5b). Several other purine analogs including 6-thioguanine, 6-thioguanosine and 6-mercaptopurine riboside were also confirmed to be active in the EGFP-IVS2-654 cell line by microscopy and RT-PCR.
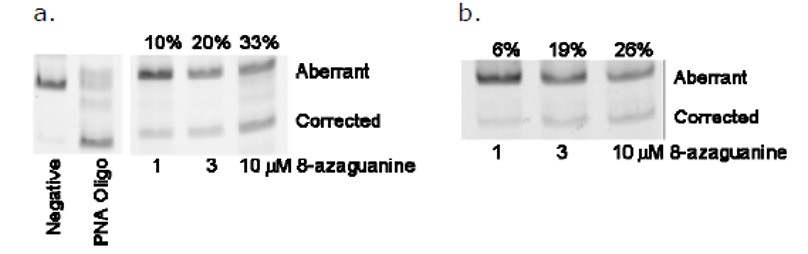
Figure 5. RT-PCR analysis of splicing modulation by 8-azaguanine. (a) In the EGFP-ISV2-654 cell line, correctly spliced mRNA significantly increased after the treatment with 8-Azaguanine, as determined by RT-PCR experiment. (b) In a β-globin-ISV2-654 cell line, the correctly spliced mRNA significantly increased after treatment with 8-azaguanine.
References
- qHTS Assay for Modulators of Hemoglobin Βeta Chain Splicing
- Schwartz E, Benz EJ, Forget BG (1995). Thalassemia Syndromes. In: Hoffman R, Benz Jr. EB, Shattil S, Furie B, Cohen H, Silberstein L, eds. Hematology Basic Principles and Practice, 586-609
- Sazani P, Kole R (2003). Therapeutic potential of antisense oligonucleotides as modulators of alternative splicing. J Clin Invest. 112(4):481-486
- Sierakowska H, Sambade MJ, Agrawal S, Kole R (1996). Repair of thalassemic human β-globin mRNA in mammalian cells by antisense oligonucleotides. Proc Natl Acad Sci U S A. 93(23):12840-12844
- Rund D, Rachmilewitz E (2005). Medical progress: β-thalassemia. New England Journal of Medicine 353(11):1135-1146


