ML127 : ALOX12 (Arachidonate 12-lipoxygenase) Inhibitor
ML127
Target Name
Arachidonate 12-lipoxygenase
Target Alias
ALOX12
Target Class
Oxygenase
Mechanism of Action
Inhibitor of ALOX12
Biological / Disease Relevance
12hLO, Inflammatory Response, Inflammatory Disease
In vitro activity
12-hLO (IC50)In vitro activity
12-hLO Cuvette Assay (IC50)In vitro activity
12-hLO Kinetic assay (Ki)Cellular Activity
Caco-2 ((Papp 10^-6 m/s @ pH 7.4)Cellular Activity
Efflux ratio (B->A)/(A->B)Inactive Control
Available
Target Information
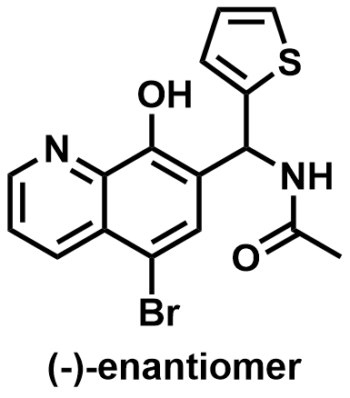
Human lipoxygenases (hLOs) are distributed among a variety of tissues and cellular locations in the body, and they are part of the first committed step in a cascade of metabolic pathways. As such, hLOs are implicated in the onset of inflammatory diseases such as cancers, heart disease and asthmas, making them an ideal target for pharmaceutical intervention. The lipoxygenase platelet-type 12-(S)-hLO has been implicated in skin diseases, diabetes, platelet hemostasis, thrombosis and cancer. Despite the potential of 12-hLO as a therapeutic target, potent and selective inhibitors of the enzyme are lacking in the patent or public literature. To date, the only known modulators of 12-hLO are either weak inhibitors or promiscuous polyphenolic compounds that inhibit several of the closely related isoymes. In this report, we describe the development of small-molecule inhibitors that exhibit nanomolar potency against 12-hLO and >50-fold selectivity over the related lipoxygenases and cyclooxygneases. Kinetic experiments indicate that this chemotype is a non-competitive inhibitor that does not reduce the active site iron. Moreover, chiral HPLC separation of several of the racemic lead molecules revealed a strong preference for the (–)-enantiomer (IC50 ~ 0.4µM) compared to > 25µM for the (+)-enantiomer, indicating a fine degree of selectivity in the active site due to chiral geometry. ML127 (CID 44460175) and its related analogs represent the most potent and selective 12-hLO inhibitors reported thus far.
Project Team
Properties

ML127
NCGC00188369-03
| Physical & chemical properties | ||||
|---|---|---|---|---|
| Molecular Weight | 407.21 | |||
| Molecular Formula | C22 H30 N6 O3 S | |||
| cLogP | 2.4 | |||
| PSA | 84.5 | |||
| Storage | -20C | |||
| Solubility | Up to 20 mM in DMSO | |||
| CAS Number | ||||
SMILES:
CC(NC(C1=C(C2=NC=CC=C2C(Br)=C1)O)C3=CC=CS3)=O
InChI:
InChI=1S/C22H30N6O3S/ c1-27(2)13-6-16-31-19-9-7-18(8-10-19)20-17-21(26-22(25-20)32(3,29)30)23-11-4-1 4-28-15-5-12-24-28/h5,7-10,12,15,17H,4,6,11,13-14,16H2,1-3H3,(H,23,25,26)
InChIKey:
MSIJJXOWLFOYIN-UHFFFAOYSA-N
Activity
Summary activity statement /
ML127 (SID 85736374; CID 44460175) is expected to be used by researchers studying inflammatory response mechanisms, human pancreatic beta-cell death during the progression of type 1 diabetes, and tumor angiogenesis. The characterized probe will be useful as a tool to study the biological role of 12-hLO in lipid- and prostaglandin-mediated signaling as related to inflammation, human platelet reactivity, progression of type 1 diabetes, and clot formation. One recent hypothesis on the inhibition of 12-hLO as a therapeutic target proposes that inhibition of 12-hLO slows down the progression of human beta-cell death in type 1 diabetes; another proposes that an inhibitor of 12hLO can be a blood-thinning agent devoid of dangerous side effects, such as the promotion of excessive bleeding. ML127 provides a potent tool compound to validate such hypotheses.
In vitro activity - Selectivity assay
| Assay | ML127 (IC50) |
|---|---|
|
12-hLO Primary biochemical assay |
0.430 uM |
|
15-hLO-1 biochemical assay |
30 uM |
|
15-hLO-2 biochemical assay |
250 uM |
Summary /
ML127 is observed to be > 70 fold selective against 12-hLO vs 15-hLO-1. It is >580 fold selective against the primary target vs 15-hLO-2.
In vitro cell-based activity - ADME Profiling
| Assay | ML127 |
|---|---|
|
aq. Kinetic sol. (PBS @ pH 7.4) |
14.5 uM |
|
Caco-2 (Papp 10^-6 m/s @ pH 7.4) |
8.8 |
|
Efflux ratio (B->A) / (A->B) |
2.3 |
|
Mouse liver microsome stability (T1/2) |
< 10 min |
|
PBS - pH 7.4 stability (% remaining after 48 hr) |
100 |
|
Mouse plasma stability (% remaining after 48 hr) |
98.3 |
Summary /
ML127’s kinetic solubility measurements were conducted at Analiza Inc. using nitrogen detection methodologies. While the Caco2 permeability, microsomal stability and mouse plasma stability experiments were conducted at Pharmaron Inc. These predictive ADME results suggest that the molecules described above should provide utility in both cell-based assays and possibility in vivo models probing the effects of 12-hLO inhibition. Importantly, this particular chemotype was found to be stable over extended time periods in both assay buffer and mouse plasma.
In vitro Activity - Mechanism of Action Studies
Summary /
As 8-hydroyquinoline are known metal chelators, the above data suggest that these inhibitors bind directly to the active site iron, which raises the possibility that these inhibitors could have the necessary redox potential to reduce the active ferric iron to the resting ferrous state, as has been reported previously. To investigate the redox potential of this chemotype, DPPH, a free radical scavenger, was incubated with the probe compound; no reduction of DPPH was observed. It should be noted that stoichiometric reduction of DPPH was achieved by the known reductive LO inhibitor, NDGA, suggesting that these 8-HQ inhibitors are not reductive in nature.
In addition to characterizing the inhibition constants, the mode of binding was investigated with steady state kinetics using the probe compound by monitoring the formation of 12-HPETE as a function of substrate and inhibitor concentration in the presence of 0.01% Triton X-100. Re-plots of KM/kcat and 1/kcat versus inhibitor concentration (Figure 2) yielded linear plots, with Kic equaling 0.8 +/- 0.1μM, which is defined as the equilibrium dissociation constant. The similar affinity of inhibitor binding to both the enzyme and the enzyme substrate complex (Kic is approximately Kiu) is a rare example of true non-competitive inhibition, which is indicative of a distal site from the catalytic site, whose inhibitor affinity is not affected by substrate binding. It should be noted that attempts to determine the reversibility of inhibition were made, but the enzyme dies too fast to allow for attempted wash-out experiments. No timedependent inhibition was displayed when ML127was incubated with 12-hLO, unlike the time-dependent inhibition seen for the bidentate catechol inhibitors against sLO-1.
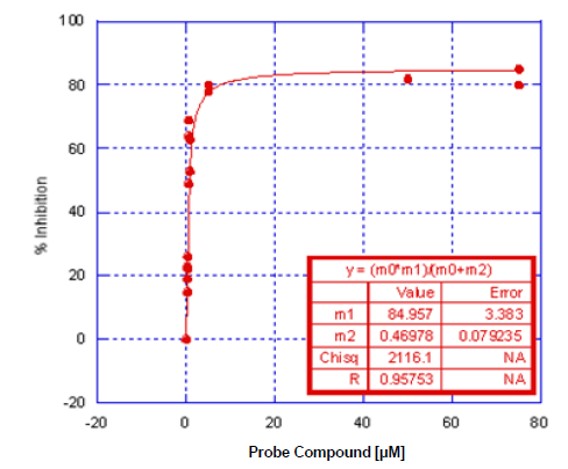
Figure 1. UV-Vis Assay Dose Response for ML127
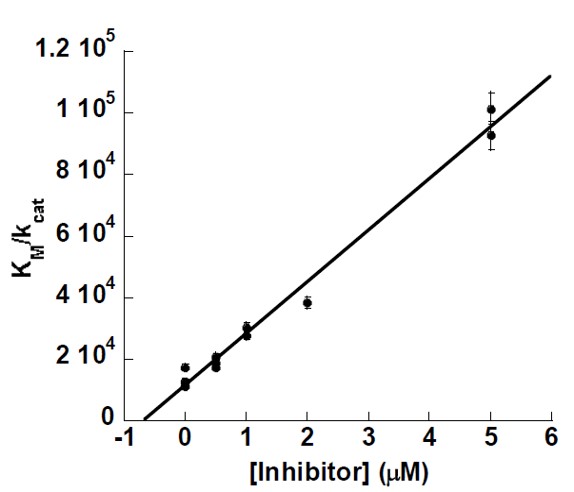
Figure 2. Steady-state kinetics data for the determination of Kic for 12-hLO with inhibitor. KM/Vmax (slope, KM units are μM) versus [Inhibitor] (μM) is the secondary replot of the inhibition data, which yielded a Kic of 0.8 +/- 0.1μM.
References
- Probe Development Summary of Inhibitors of 12-hLO (12-human lipoxygenase)
- Rai G, Jadhav A, Schultz L, et al. Selective Small Molecule Inhibitors of 12-Human Lipoxygenase (12-hLO) 2009 Nov 30 [Updated 2011 Mar 25]. In: Probe Reports from the NIH Molecular Libraries Program [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2010
- Luci D, Jameson JB II, Yasgar A, et al. Discovery of ML355, a Potent and Selective Inhibitor of Human 12-Lipoxygenase. 2013 Apr 12 [Updated 2014 Sep 18]. In: Probe Reports from the NIH Molecular Libraries Program [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2010


