ML154 : NPSR1 (Neuropeptide-S Receptor) Antagonist

ML154
Target Name
Neuropeptide-S Receptor
Target Alias
NPSR1
Target Class
G-protein Coupled Receptor
Mechanism of Action
Antagonist of NPSR1
Biological / Disease Relevance
NPS/NPSR pharmacology; NPS's role in sleep, anxiety, food intake, and addiction.
Cellular activity
NPSR Ca2+ bioassay (IC50)Cellular activity
NPSR cAMP bioassay (IC50)Cellular activity
[125I]Y10-hNPS displacement bioassay (EC50)Target Information
NPSR is a G-protein coupled receptor that was first described by Sato and co workers in 2004 (Sato 2002). Subsequently it was deorphanized by Xu, who described the structure of NPS as a 20-amino acid endogenous ligand which binds to NPSR (Xu 2004). Later on, the same group also detailed the distribution and expression of NPS precursor mRNA and NPSR mRNA in various regions of the brain (Xu 2007). Additionally, it was shown that central administration of NPS promotes hyperlocomotion and wakefulness in various rodent models, implicating the importance of this circuitry in the control of sleep, stress, anxiety and arousal (Pape 2009, Okamura 2008). Additionally, two polymorphisms of NPSR, Ile107 from Asn107 and a C-terminal splice variant, were linked to asthma (Laitinen 2004). Moreover, Reinscheid et al. detailed the NPS/NPSR pharmacology via stable cell lines expressing NPSR and these variants (Reinscheid 2005), characterizing a binding assay which monitored the displacement of radiolabeled 125I-NPS ligand by the cognate NPS ligand. They also described three separate functional assays which increased calcium mobilization, cAMP formation, and p42/p44 MAPK phosphorylation in a dose-dependent manner on addition of NPS. This work suggested that the receptor is coupled to signaling through the Gq, Gs and MAPK pathways, and also shows that the I107N NPRS variant displayed an increased functional potency in all these assays but not in binding affinity. Recently, the possible importance of the extracellular loop one, where this SNP is located, has been highlighted in the biogenesis and function of NPSR (Clark 2010).
Properties

ML154
NCGC00185684
| Physical & chemical properties | ||||
|---|---|---|---|---|
| Molecular Weight | 544.0738 g/mol | |||
| Molecular Formula | C29H26BrN2PS | |||
| cLogP | ||||
| PSA | 41.1 Ų | |||
| Storage | ||||
| Solubility | 10 mM in DMSO; > 150 uM in PBS | |||
| CAS Number | 1345964-89-7 | |||
SMILES:
CC1=C(P(C2=CC=CC=C2)(C3=CC=CC=C3)=S)N4C=CC=CC4=[N+]1C/C=C/C5=CC=CC=C5
InChI:
1S/C29H26N2PS.BrH/c1-24-29(32(33,26-17-7-3-8-18-26)27-19-9-4-10-20-27)31-22-12-11-21-28(31)30(24)23-13-16-25-14-5-2-6-15-25;/h2-22H,23H2,1H3;1H/q+1;/p-1/b16-13+;
InChIKey:
CJAQCMBWGUOBIX-ZUQRMPMESA-M
Activity
Summary activity statement /
ML154 (SID 87796314; CID 46930969) is a potent NPSR antagonist having an imidazo-pyridinium molecular core and an unusual, yet stable phosphorothioyl species. Synthetic methodology, SAR for both Gq and Gs coupled functional pathways and activity of our most potent compound in a NPS displacement assay are presented. ML154 is the most potent compound yet reported and has promising microsomal stability compared to other lead NPSR antagonists disclosed in the literature. In vivo rodent pharmacokinetic experiments show that a 10 mpk dose administered IP may provide sufficient exposure in brain to see functional antagonism for many hours. This probe completely antagonizes NPS activation of the NPS/NPSR neurocircuitry in a food intake rat model using intracerebroventricular (icv) administration. ML154 can be used as a tool molecule by biologists interested in understanding NPS/NPSR pharmacology and the role of NPSR antagonism in sleep, anxiety, food intake, and addiction.
Cellular activity - Selectivity Profiling
| Target | ML154 (IC50) |
|---|---|
|
NPSR Ca2+ assay |
1 nM |
|
NPSR |
45 nM |
|
V1B Vasopressin Receptor (Anti-target) |
> 57000 nM |
|
[125I]Y10-hNPS displacement |
1 nM |
Summary /
ML154 is found to have > 100 fold selective against the NPSR with nanomolar potency (Figure 1) vs a vasopressin receptor V1B that has 27% identity with NPSR. In addition the probe is profiled against 8 other targets (Table 1).
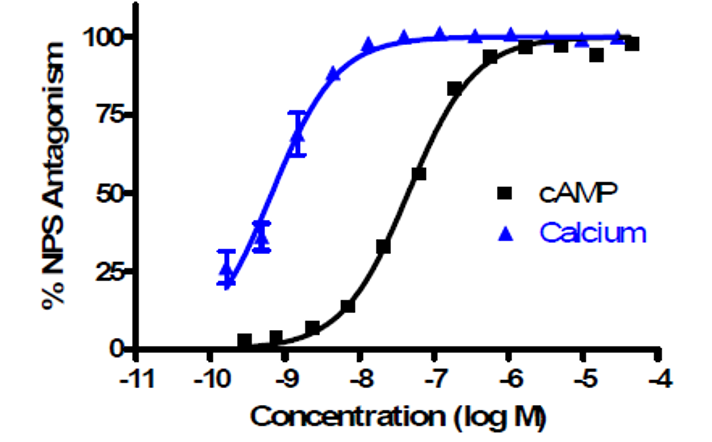
Figure 1: Concentration-response of the probe molecule, CID 46930969, in functional assays of NPSR antagonism: cAMP signaling IC50 = 45nM (black) and calcium signaling IC50 = 1nM (blue), 4 replicates each.
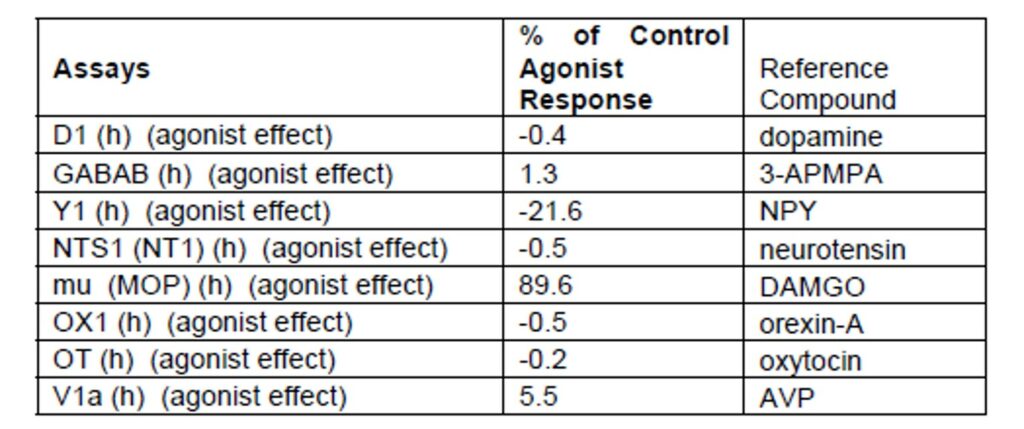
Table 1: The probe, CID 46930969, was profiled against 8 other targets at @ 10 μM at Cerep®. 90% activity was discovered in the mu agonist displacement assay.
Cellular activity - Mu Receptor Assay
Summary /
IC50 determination in a radiolabeled displacement assay determined that the ML154’s activity against the mu-receptor was >500-fold less potent compared to the IC50 in the 125I-NPS displacement assay (Table 2 and Figure 2).
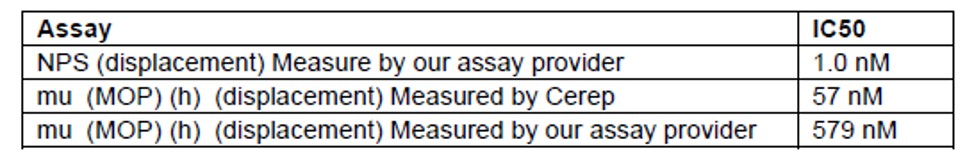
Table 2. ML154 activity: NPSR vs. mu-opioid receptor (MOP). An IC50 of 57 nM in the mu-agonist displacement assay at Cerep® is observed. Although there is a 10-fold shift in potency between the MOP activities between Cerep and our assay provider, we have to be cognizant of this activity during in vivo studies.
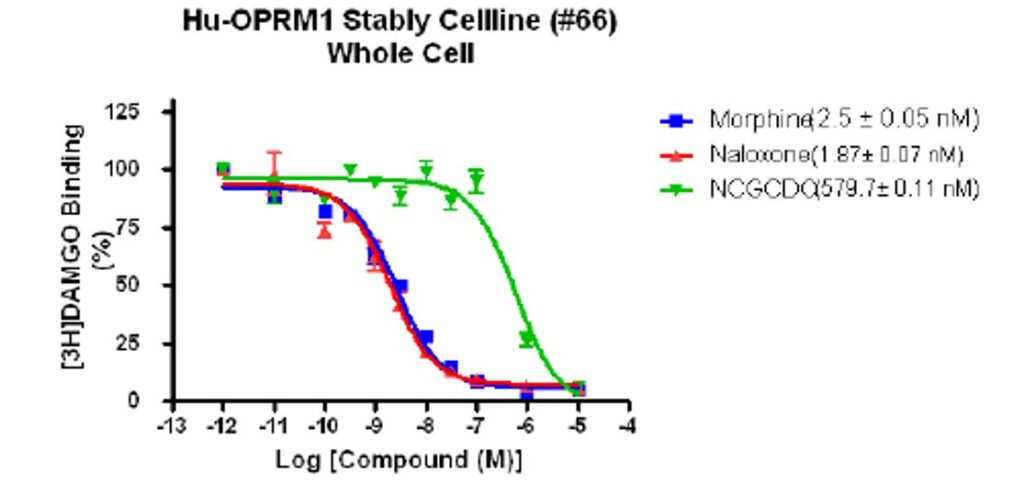
Figure 2: CID 46930969 mu receptor activity (assay provider).
Cellular activity - GRCR panel
Summary /
ML154 (CID 46930969) is profiled at 10 uM against a GRCR panel of 55 targets at Cerep® in binding assays. IC50s against targets which recorded > 90% inhibition at 10 uM (Patnaik 2010).
In vivo activity - PK Profiling
Summary /
The stability in mouse liver microsomes spurred an evaluation of the pharmacokinetics of ML154 (CID 46930969) at a 10 mpk doses in mice (Tables 3 and 4). There were no signs of adverse effects with this dose. The mice appeared healthy with compound concentrations ranging from 0.05 to 1.5 uM in the plasma and 30 to 93 nM in the brain. The compound concentration in the brain was still above the in vitro IC50 at 24h. By comparison, the reported in vivo rat PK of a prior art compound from Merck at 30 mg/kg IP dose reveals the following tissue levels at 0.5 h: Plasma/Brain/CSF (nM) = 4344, 1690, 101 and at 2.0 h: Plasma/Brain/CSF (nM) =2297, 665, 45. SHA68 (1b) reaches the following concentrations in mice after a 50 mpk IP dose at 15 min: Plasma/Brain, 88, 6.3 uM and at 2.0 h: 1.1, 2.1 uM. Thus, these two compounds appear to be rapidly cleared from the plasma and the brain, and this is consistent with the in vitro microsomal stability data generated by NCGC. ML154 seems to maintain steady concentrations well above the IC50 for 24 h when it is administrated IP at a dose of 10 mg/kg. This may be critical for in vivo activity, as SHA68 (prior art compound) only shows partial efficacy in a hyperlocomotion mouse model (Patnaik 2010).
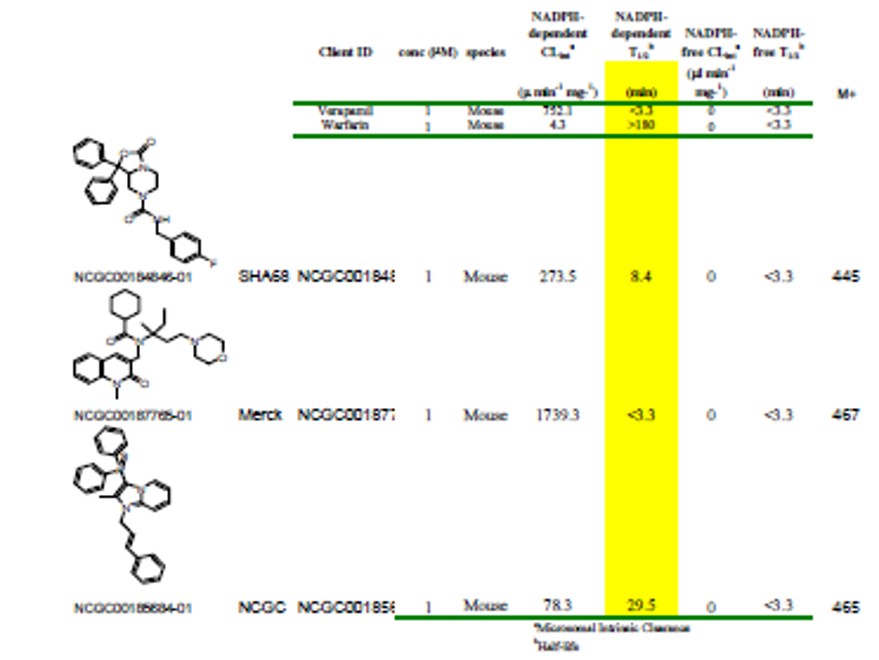
Table 3. Comparison of the microsomal stability of ML154 and 2 other known NPSR antagonists.
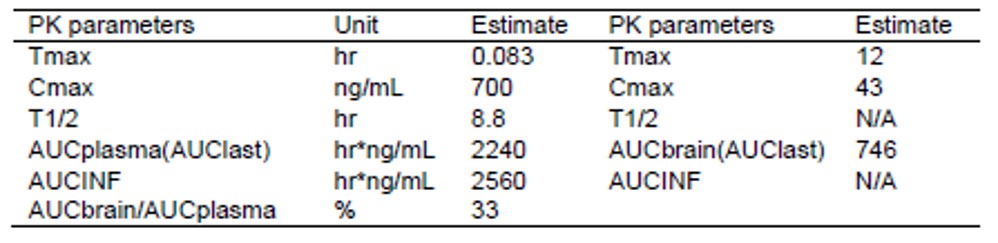
Table 4. ML154 Mouse PK Paramaters and Curve for 10 mpk IP dose.
In vivo activity - Rodent food intake suppression reversal assay.
Summary /
Activity of ML154 in animal (rodent) model, where hyperactivation of the NPS signaling causes a suppression of food intake, is assessed. Rats with intracranial implants were habituated to a sweetened diet. The food intake was measured at 15, 30, and 60 min in a control group and in animals where NPS was administered icv (Figure 3). Animals were balanced between groups so that the pre-test baseline intake was matched. The NPS induced reduction in food intake (orange line) compared to controls (green) was completely reversed by intracerebroventricular (icv) administering of ML154 (10 ug) prior to NPS administration (6-8 animals/group).
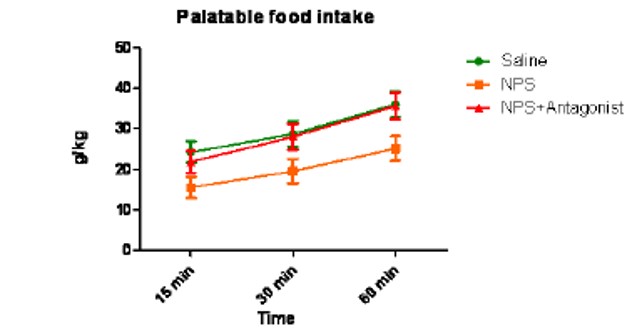
Figure 3. ML154 Food Intake icv Experiment showed reversal of the food intake suppression.
References
- PubChem link: qHTS Assay for Antagonists of the Neuropeptide S Receptor: cAMP Signal Transduction
- Patnaik S, Marugan J, Liu K, et al. Identification of Small Molecule Antagonists of the Neuropeptide-S Receptor. 2010 Mar 19 [Updated 2010 Dec 16]. In: Probe Reports from the NIH Molecular Libraries Program [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2010-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK51966/
- Huh JR, Englund EE, Wang H, et al. Identification of Potent and Selective Diphenylpropanamide RORγ Inhibitors. ACS Med Chem Lett. 2013;4(1):79-84. doi:10.1021/ml300286h
- Sato, S. S. Y.; Miayajima, N.; Yoshimura, K. Novel G-protein coupled receptor protein and DNA thereof, World Patent Application WO 02/31145 A1. 2002
- Xu YL, Reinscheid RK, Huitron-Resendiz S, Clark SD, Wang Z, Lin SH, Brucher FA, Zeng J, Ly NK, Henriksen SJ, de Lecea L, Civelli O. Neuropeptide S: a neuropeptide promoting arousal and anxiolytic-like effects. Neuron. 2004 Aug 19;43(4):487-97. doi: 10.1016/j.neuron.2004.08.005. PMID: 15312648
- Xu YL, Gall CM, Jackson VR, Civelli O, Reinscheid RK. Distribution of neuropeptide S receptor mRNA and neurochemical characteristics of neuropeptide S-expressing neurons in the rat brain. J Comp Neurol. 2007 Jan 1;500(1):84-102. doi: 10.1002/cne.21159. PMID: 17099900.
- Pape HC, Jüngling K, Seidenbecher T, Lesting J, Reinscheid RK. Neuropeptide S: a transmitter system in the brain regulating fear and anxiety. Neuropharmacology. 2010 Jan;58(1):29-34. doi: 10.1016/j.neuropharm.2009.06.001. Epub 2009 Jun 10. PMID: 19523478; PMCID: PMC2784192
- Okamura N, Reinscheid RK. Neuropeptide S: a novel modulator of stress and arousal. Stress. 2007 Aug;10(3):221-6. doi: 10.1080/10253890701248673. PMID: 17613937
- Laitinen T, Polvi A, Rydman P, Vendelin J, Pulkkinen V, Salmikangas P, Mäkelä S, Rehn M, Pirskanen A, Rautanen A, Zucchelli M, Gullstén H, Leino M, Alenius H, Petäys T, Haahtela T, Laitinen A, Laprise C, Hudson TJ, Laitinen LA, Kere J. Characterization of a common susceptibility locus for asthma-related traits. Science. 2004 Apr 9;304(5668):300-4. doi: 10.1126/science.1090010. PMID: 15073379
- Reinscheid RK, Xu YL, Okamura N, Zeng J, Chung S, Pai R, Wang Z, Civelli O. Pharmacological characterization of human and murine neuropeptide s receptor variants. J Pharmacol Exp Ther. 2005 Dec;315(3):1338-45. doi: 10.1124/jpet.105.093427. Epub 2005 Sep 6. PMID: 16144971
- Clark SD, Tran HT, Zeng J, Reinscheid RK. Importance of extracellular loop one of the neuropeptide S receptor for biogenesis and function. Peptides. 2010 Jan;31(1):130-8. doi: 10.1016/j.peptides.2009.10.015. Epub 2009 Oct 27. PMID: 19874863; PMCID: PMC2814945.


