ML198 : GBA (Glucocerebrosidase (N370S mutant)) Modulator
ML198
Target Name
Glucocerebrosidase (N370S mutant)
Target Alias
GBA
Target Class
Hydrolase
Mechanism of Action
Modulator of GBA
Biological / Disease Relevance
Gaucher; ER-lysosomal trafficking; chaperone of glucocerobrosidase
In vitro activity
N370S GCase tissue homogenate (EC50)In vitro chaperone activity
N370S GCase fibroblast (EC50)Target Information
Gaucher disease is a rare genetic lysosomal storage disease characterized by a loss of function in the glucocerebrosidase (GCase) enzyme, which is responsible for hydrolyzing glucocerebroside (GC) in the lysosome. When cells die, macrophages use GCase to break down GC, a major constituent of cell walls. With deficient functional GCase, GC accumulates within the lysosome, giving rise to the appearance of bloated Gaucher cells; this is a hallmark of the disease. Certain mutated GCase proteins, after production in the endoplasmic reticulum (ER), do not fold properly and are degraded via the proteasome pathway instead of being transported to the lysosome. One therapeutic strategy is to develop small molecule chaperones, which upon binding to GCase ensure proper folding and subsequent transport of the mutant protein to the lysosome, where it can resume activity. The main challenge in the development of molecular chaperones for Gaucher disease is that all of the previously described chaperones are inhibitors of the enzyme. This complicates their clinical development, because it is difficult to generate an appropriate in vivo exposure at which a compound exhibits chaperone activity, but does not inhibit the enzyme’s function. Using high throughput screening, we have identified two chemical series that do not inhibit the enzyme’s action, but can still facilitate its translocation to the lysosome as measured by immunostaining of glucocerebrosidase in patient fibroblasts. These chemical series are exemplified by ML198 and ML266. These compounds serve as starting points to develop a novel approach towards small molecule treatment for patients suffering from Gaucher disease.
Project Team
Properties

ML198
NCGC00188758
| Physical & chemical properties | ||||
|---|---|---|---|---|
| Molecular Weight | 290.32 g/mol | |||
| Molecular Formula | C17H14N4O | |||
| cLogP | 2.2 | |||
| PSA | 59.3 A^2 | |||
| Storage | -20 | |||
| Solubility | ||||
| CAS Number | 1380716-06-2 | |||
SMILES:
CC1=NC2=C(C(NC3=CC=C(C#C)C=C3)=O)C=NN2C(C)=C1
InChI:
InChI=1S/C17H14N4O/c1-4-13-5-7-14(8-6-13)20-17(22)15-10-18-21-12(3)9-11(2)19-16(15)21/h1,5-10H,2-3H3,(H,20,22)
InChIKey:
HVZPJBKUFRREQC-UHFFFAOYSA-N
Activity
Summary activity statement /
The class of pyrazolopyrimidines and salicylic acid derivatives function as activator of GCase (glucocerebrosidase or acid beta glucosidase) in a functional N370S (the most common mutation in Gaucher disease) spleen homogenate assay, and show chaperone activity in a translocation assay using patient-derived fibroblasts. ML198 (CID 46907762; SID 99368009), due to the lack of its inhibitory activity, does not have the risk of inhibiting enzyme function after transport to the lysosome. Furthermore, unlike previous iminosugar chaperones that may have had side effects because of a lack of selectivity against other glucosidases, this compound is also quite selective for GCase activity. Thus, this first-in-class non-inhibitor chaperones can be used as tools to further evaluate their therapeutic potential in various models of Gaucher disease. This compound also demonstrates a lack of activity towards substrate hydrolysis of the structurally related acid alpha glucosidase and alpha galactosidase, thus appears to be selective towards GCase.
In vitro activity - Selectivity Assay
| ML198 (EC50) | |
|---|---|
|
N370S GCase |
0.4 uM |
|
Acid alpha-glucosidase |
> 57 uM |
|
Beta-glucosidase |
Inactive |
|
Beta-galactosidase |
Inactive |
Summary /
ML198 was profiled against β-glucosidase and β-galactosidase. It is found to be inactive against these glucosidases, and > 100 fold selective towards the GCase vs. alpha-glucosidase.
In vitro activity - ADME profiling
| ML198 | |
|---|---|
|
Aqueous Kinetic Solubility (uM) |
2.3 |
|
Mouse Liver Micorsomal Stability +NADPH (% remaining after 60 min) |
45 |
|
Mouse Liver Microsomal Stability - NADPH (% remaining after 60 min) |
42 |
|
Caco-2 Permeability (A to B) (10^-6 cm s^-1) |
6.0 |
|
Caco-2 Permeability (B to A) (10^-6 cm s^-1) |
2.0 |
|
Caco-2 Permeability (Efflux ratio) |
0.3 |
Summary /
The microsomal stability and Caco-2 permeability data for ML198 showed a low water solubility, a modest permeability in a Caco-2 assay. Analysis of the metabolic stability after a 60 min-incubation in mouse liver microsomes revealed rapid clearance.
In vitro cellular activity - Immunofluorescence Staining
Summary /
With DMSO treatment, GCase remains largely in the nucleus, and only 5% of the cells indicated translocation of GCase to the lysosome. We tested the known iminosugar isofagomine and observed translocation in 17% of the cells at 5 μM. At 5 μM, ML198 and ML266 give very convincing translocation visually, and their percentage of cell translocations are 20 and 15%, respectively. The high magnification images in Figure 2 (the yellow color in the bottom right quadrant) clearly show the colocalization
of CGase in the periphery of the cell, where the lysosome is located. The colocalization was also observed to increase in a dose-dependent manner, demonstrating the chaperone behavior of the ML198.
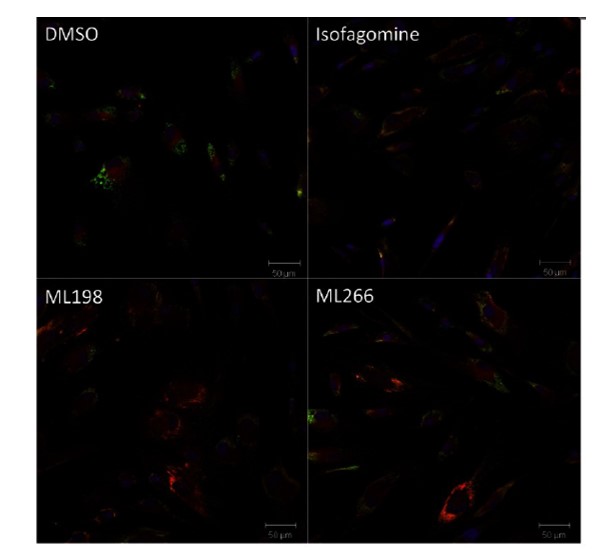
Figure 1. Human Fibroblast experiment: the nucleus is stained with DAPI (blue), the lysosome is stained with LAMP-2 (green), and GCase is visualized through a GBA antibody (red).
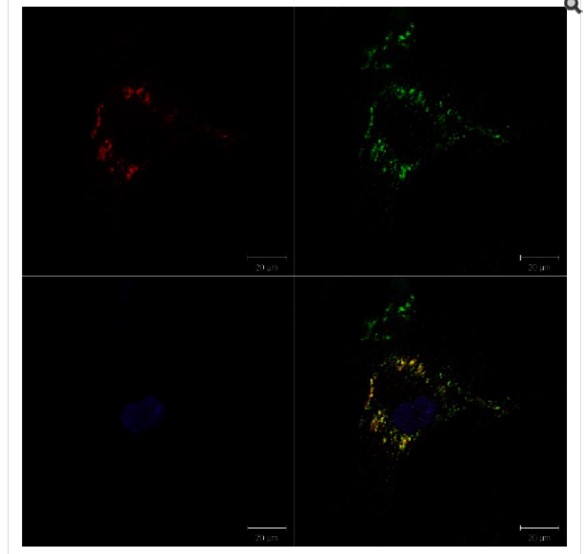
Figure 2. High magnification image of translocation experiment in a single cell with with 5 μM ML198. Localization of GCase (red) into the lysososomes (green) is evident by the yellow color in the overlapped image. in the bottom right quadrant.
References
- qHTS Assay for Inhibitors and Activators of N370S glucocerebrosidase as a Potential Chaperone Treatment of Gaucher Disease: Summary
- Rogers S, Patnaik S, Schoenen F, et al. Discovery, SAR, and Biological Evaluation of Non-inhibitory Chaperones of Glucocerebrosidase. 2012 Mar 27 [Updated 2013 Mar 7]. In: Probe Reports from the NIH Molecular Libraries Program [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2010
- Sardi SP, Cheng SH, Shihabuddin LS. Gaucher-related synucleinopathies: the examination of sporadic neurodegeneration from a rare (disease) angle. Prog Neurobiol. 2015;125:47-62. doi:10.1016/j.pneurobio.2014.12.001
- Aflaki E, Stubblefield BK, Maniwang E, et al. Macrophage models of Gaucher disease for evaluating disease pathogenesis and candidate drugs. Sci Transl Med. 2014;6(240):240ra73. doi:10.1126/scitranslmed.3008659


