ML201 : GAA (Lysosomal Alpha-glucosidase) Inhibitor
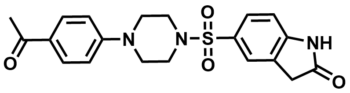
ML201
Target Name
Lysosomal Alpha-glucosidase
Target Alias
GAA
Target Class
Glucosidase
Mechanism of Action
Inhibitor of GAA
Biological / Disease Relevance
Pompe Disease, Lysosomal Storage Disorders (LSD), Diabetes Mellitus Disorder Type 2
In vitro activity
Acid alpha-glucosidase bioassay (IC50)In vitro activity
Acid alpha-glucosidase cleavage of glycogen (IC50)Target Information
Acid alpha-glucosidase is an enzyme that catalyzes the exohydrolysis of 1,4-alpha-glucosidic linkages to release glucose. Alpha-glucosidase inhibitors are not only useful for limiting the impact of carbohydrate consumption on blood glucose levels in patients with diabetes mellitus type 2, but may also be useful as small molecule chaperones for correcting the misfolding and mistrafficking of mutant alpha-glucosidase in Pompe disease and or Lysosomal Storage Disorders (LSD). These small molecule chaperones are a promising new therapeutic approach that are able to impact the accumulation of functional mutant in the Endoplasmic Reticulum, increasing translocation and ameliorating the disease phenotype.
Here, we present the discovery, structure activity relationship (SAR) and initial biological results of a new series of acid alpha glucosidase non-iminosugar inhibitors with chaperone capacity, as exemplified by ML201 (SID 49731601; CID 20969430). In addition, these compounds could potentially be useful for Diabetes type 2 treatment.
Properties

ML201
NCGC00187771
| Physical & chemical properties | ||||
|---|---|---|---|---|
| Molecular Weight | 399.46 g/mol | |||
| Molecular Formula | C20H21N3O4S | |||
| cLogP | 2.0501 | |||
| PSA | 86.79 Ų | |||
| Storage | ||||
| Solubility | 10 mM in DMSO; 20 uM in PBS pH 7.4 | |||
| CAS Number | ||||
SMILES:
CC(C1=CC=C(N2CCN(S(=O)(C3=CC4=C(NC(C4)=O)C=C3)=O)CC2)C=C1)=O
InChI:
1S/C20H21N3O4S/c1-14(24)15-2-4-17(5-3-15)22-8-10-23(11-9-22)28(26,27)18-6-7-19-16(12-18)13-20(25)21-19/h2-7,12H,8-11,13H2,1H3,(H,21,25)
InChIKey:
IOWHNNUEAIMNRP-UHFFFAOYSA-N
Activity
Summary activity statement /
ML201 is a new class of inhibitors of alpha-glucosidase based upon a substituted 5-(4-phenylpiperazin-1-ylsulfonyl)indolin-2-one scaffold identified from a qHTS campaign. We investigate the synthesis of these agents, structure activity relationships (SAR), analysis of activity using a functional, thermal destabilization assay and ADME properties, including in vivo pharmacokinetic analysis of the probe compound. These agents represent a novel non-iminosugar chemotype of inhibitors of alpha-glucosidase, and may be useful as chaperones of acid alpha-glucosidase for the treatment of Pompe disease or as enzyme inhibitors in the context of diabetes mellitus disorder type 2.
In vitro activity - Selectivity and Cytotoxicity Assay
| Bioassay | ML201 (IC50) |
|---|---|
|
Acid alpha glucosidase |
520 nM |
|
Acid alpha - glucosidase cleavage of glycogen |
330 nM |
|
beta - glucosidase (Anti-Target) |
> 59400 nM |
|
alpha - galactosidase (Anti-Target) |
> 51100 nM |
Summary /
ML201 is found to have > 100 fold selective against the acid alpha-glucosidase vs. beta-glucosidase and alpha-galactosidase. ML201 was further assayed using purified enzyme with its native substrate, glycogen. Glycogen from bovine liver was used as the substrate and recombinant human alpha-glucosidase as the enzyme preparation. The compound had a potency of 330 nM in this assay, consistent with its activity in the primary screening assay.
In vitro activity - Functional assay: Thermal destabilization of alpha-glucosidase
Summary /
ML201’s ability to prevent loss of enzyme function following thermal destabilization of alpha-glucosidase is tested. Briefly, hydrolytic enzymes lose their catalytic activity over time after exposure to elevated temperatures below their melting point. This is due to progressive denaturation and/or aggregation of the protein from solution. Warming acid alpha glucosidase to 66ºC for 60 minutes reduces enzyme activity by about 75%. Compounds that bind to the enzyme may prevent this loss of activity. Compounds that prevent thermal destabilization have also been shown to promote cellular folding and translocation, and therefore have the potential capacity of being chaperone molecules. Results for ML201 (green and blue) demonstrate that this compound can stabilize alpha-glucosidase.
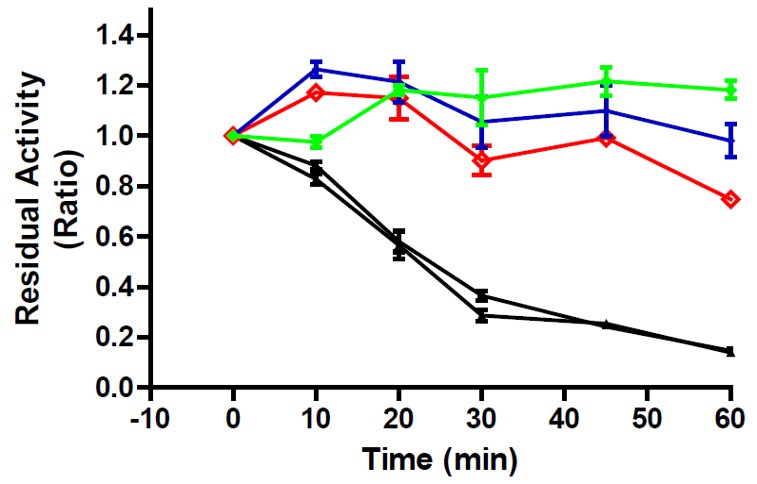
Figure 1. Thermal stabilization of alpha-glucosidase functional activity following incubation at destabilizing temperatures. Time indicates length of incubation at 66ºC. Ratio is the ratio of enzymatic activity after incubation at 66ºC compared to no high temperature incubation. Vehicle (DMSO) control experiments in black, the iminosugar DNJ (2.5 μM; CID 1374; SID 50111045) in red, and the MLS001030468 sample of ML201 (50μM; ML201) in green, and a re-synthesized sample of ML201 (50μM; ML201) in blue.
Cellular Activity - GAA ER-lysosome translocation.
Summary /
For measuring the capacity of our compounds to activate ER-lysosome translocation of GAA, we follow the previously described protocol by treating Pompe fibroblast with potential chaperone compounds for five days, followed by immunostaining GAA (green) and Cathepsin D (red) as lysosomal marker, repetitive wash and image analysis.
ML201 and two analogs (CID 36649951 and CID 44825300) were selected for further evaluation in the translocation assay using fibroblasts with p.Y455C/p.G638W GAA mutations. All of the compounds were toxic for cells carrying the p.Y455C/p.G638W mutations. At low concentration (1 μM), some of the cells survived but they looked very sick (big holes in the cells are indicated by the arrows in Figure 2 and enlarged lysosomes). One potential explanation is that the GAA activity of this cell line with p.Y455C/p.G638W mutations is very low, and therefore the addition of an inhibitor could completely eliminate the function of the enzyme, having an impact in cell survival. To test this hypothesis, we decided to evaluate the same compounds in wild type fibroblast. Our probe molecule (ML201) seemed to be toxic even for wild type, although the other compounds provided better results. CID 36649951 is found to up-regulate Cathepsin D and translocates wt GAA to lysosomes, increasing the percent of cells having GAA in the lysosome (from 15% to 30%). At the same time, the size of lysosomes increase, which generally is not considered healthy for a cell (Figure 3). CID 44825300 upregulates Cathepsin D and translocates wt GAA to lysosomes, increasing the percent of cells having GAA in the lysosome (from 15% to 50%) without increasing the size of lysosomes (Figure 4). Therefore, this compound could be considered an excellent chaperone for wt GAA. Interestingly, this compound does not disclose any inhibitory activity against wt GAA in our functional assay probing that the inhibitory activity and the translocation capacity do not correlate directly.
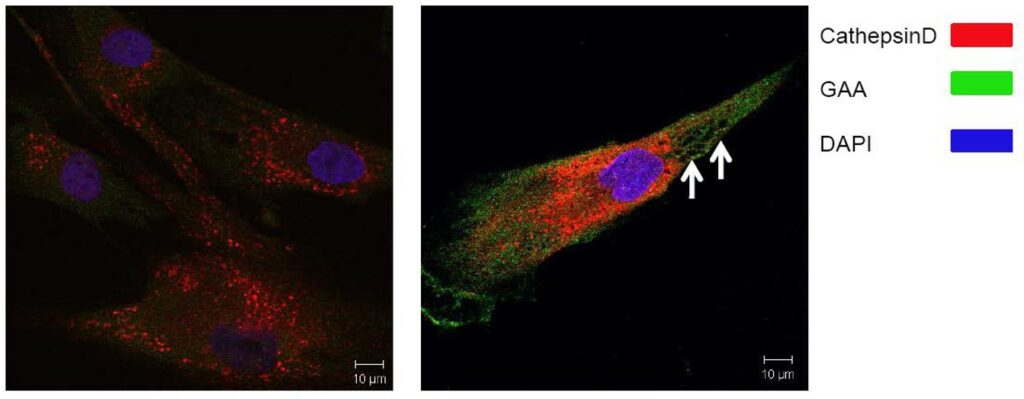
Figure 2. Representative image of p.Y455C/p.G638W GAA fibroblast treated with selective compounds.
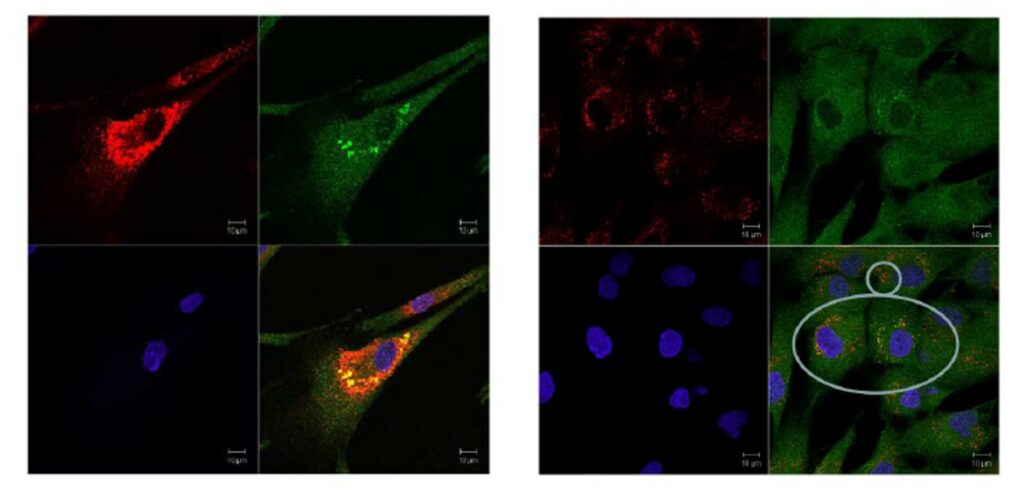
Figure 3. Treatment of wt fibroblast with 15 μM (left panel) and of 5 μM (right panel) CID 36649951.
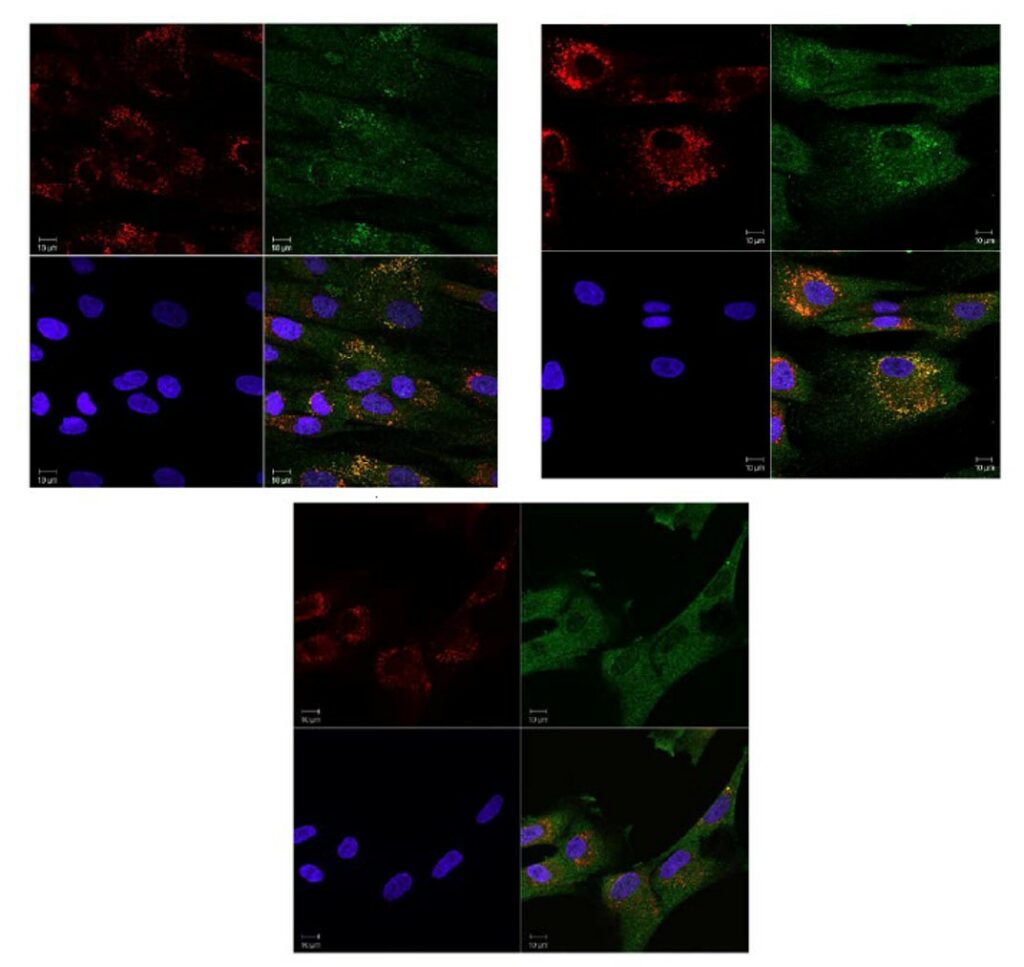
Figure 4. Treatment of wt fibroblast with 15 μM (first row, left panel), of 5 μM (first row, right panel) and 1 μM (second row, right panel) of CID 44825300.
In vitro and vivo activity - ADME and PK Profiling
Summary /
ML201 has a low intrinsic clearance and a long in vitro half life, with more than 60% of the mean parent compound remaining after one hour of incubation with mouse microsomes. The transformation that does occur is NADPH-dependent, which indicates that the major metabolic process is mostly through the cytochrome P450 dependent oxidation. Caco-2 data in Table 1 indicates that this probe compound ML201 displayed very good cell permeability in both directions (apical to basolateral (A-B) and basolateral to apical (B-A)), However, the B-A permeability was higher than the corresponding A-B permeability (efflux ratio = 1.9, [B-A/A-B]). This slight preference indicates active efflux by P-glycoprotein and other ABC transporters, although the value of the efflux ratio is reasonable and the permeability in every direction is high, therefore expecting in vivo saturation of the efflux transport.
The plasma and intestine concentrations of ML201 in male Swiss Albino Mice (after single oral gavage administration of ML201 at a dose of 30 mg/kg) displayed a very high concentration in intestine, greater than it was in plasma, confirming our hypothesis from the Caco-2 data. The pharmacokinetic parameters of ML201 in plasma and intestine were calculated and AUC(0-t) of plasma and intestine used for determination of intestine to plasma ratio are summarized in Table 2 and 3.
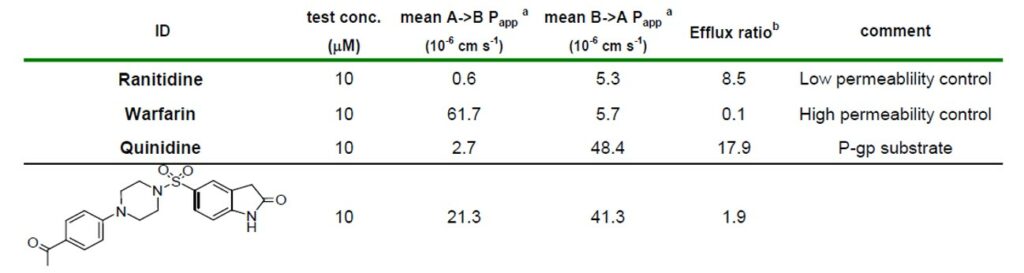
Table 1: Caco-2 permeability assays. ML201 shown as last row in table.
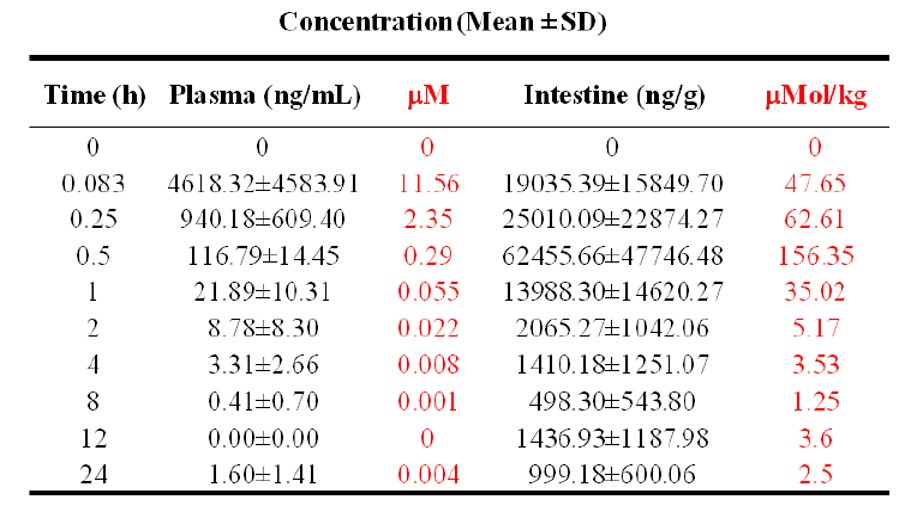
Table 2: Mice plasma and intestine levels for probe compound ML201 following oral gavage administration at 30 mg/kg in mice.
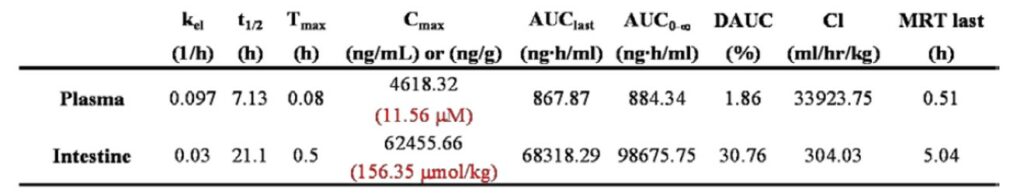
Table 3: Mice pharmacokinetic parameters for probe compound ML201 following oral gavage administration at 30 mg/kg.
References
- PubChem link: Quantitative High-Throughput Screen for Inhibitors and Activators of Human alpha-Glucosidase as a Potential Chaperone Treatment of Pompe Disease: Summary
- Xiao J, Marugan JJ, Zheng W, et al. 5-(4-(4-Acetylphenyl)piperazin-1-ylsulfonyl)indolin-2-one Analogs as Inhibitors of Acid alpha-Glucosidase for Potential Chaperone Treatment of Pompe Disease or Intervention for Diabetes Mellitus Type 2. In: Probe Reports from the NIH Molecular Libraries Program. Bethesda (MD): National Center for Biotechnology Information (US); October 28, 2010.


