ML238 : P. falciparum (Plasmodium falciparum) Inhibitor
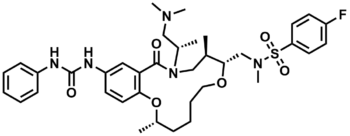
ML238
Target Name
Plasmodium falciparum
Target Alias
P. falciparum
Target Class
Parasite Plastid
Mechanism of Action
Inhibitor of P. falciparum
Biological / Disease Relevance
Malaria, Anti-parasitic drug, Dd2 and 3D7 P. falciparum
In vivo activity
P. falciparum Dd2 (IC50)In vivo activity
P. falciparum 3D7 (IC50)Target Information
Malaria, the most devastating of the parasitic diseases, afflicts an estimated 300-500 million people worldwide and results in more than 800,000 deaths annually. Several parasites in the genus Plasmodium infect humans, but Plasmodium falciparum has the most significant lethality. Drug resistance has compromised the efficacy of existing drugs. New antimalarial agents, especially agents that work against new targets in P. falciparum, are needed. As part of efforts to identify novel inhibitors of P. falciparum, we initiated a quantitative high-throughput screening (qHTS) campaign against the 3D7 line of P. falciparum. The robust whole parasite viability assay was screened against the Molecular Libraries Small Molecule Repository (MLSMR) as well as the Diversity-Oriented Synthesis (DOS) informer I library (approximately 8,000 compounds), which is a subset of the Broad Institute DOS library. From these efforts, we have identified a unique and novel structural class of antimalarial compounds from the Broad Institute DOS Informer I library. The probe (ML238) displayed picomolar activity in the SBYR live/dead assay against 3D7 and Dd2 P. falciparum. This activity was recapitulated in the luciferase assay, albeit at single-digit nanomolar activity, while showing no apparent mammalian cell cytotoxicity. The probe is in a novel structural class in the antimalarial field with unknown mechanism of action; therefore, this probe will be highly useful to the malaria research community in identifying new targets or developing new drug classes.
Properties

ML238
CAM-9-027
| Physical & chemical properties | ||||
|---|---|---|---|---|
| Molecular Weight | 711.9 g/mol | |||
| Molecular Formula | C37H50FN5O6S | |||
| cLogP | 5.3 | |||
| PSA | 129 Ų | |||
| Storage | ||||
| Solubility | ||||
| CAS Number | ||||
SMILES:
C[C@H](N1C[C@H]([C@@H](OCCCC[C@@H](OC2=C(C1=O)C=C(NC(NC3=CC=CC=C3)=O)C=C2)C)CN(S(=O)(C4=CC=C(F)C=C4)=O)C)C)CN(C)C
InChI:
1S/C37H50FN5O6S/c1-26-23-43(27(2)24-41(4)5)36(44)33-22-31(40-37(45)39-30-13-8-7-9-14-30)17-20-34(33)49-28(3)12-10-11-21-48-35(26)25-42(6)50(46,47)32-18-15-29(38)16-19-32/h7-9,13-20,22,26-28,35H,10-12,21,23-25H2,1-6H3,(H2,39,40,45)/t26-,27+,28+,35+/m1/s1
InChIKey:
BKTXRPJVVXUPPO-PNCWTNKOSA-N
Activity
Summary activity statement /
Malaria, the most devastating of the parasitic diseases, afflicts an estimated 300-500 million people worldwide and results in more than 800,000 deaths annually. More than 85% of all malaria-related mortality is among children in sub-Saharan Africa. Drug resistance has compromised the efficacy of existing drugs, and new antimalarial agents, especially those that work against new targets in Plasmodium falciparum, are needed. ML238 (SID 104179292; CID 49849912) represents a novel structural class for small molecules with antimalarial properties and may possess promise as a novel antimalarial. Further, this chemotype may provide insight into new molecular target(s) for development of additional antimalarial agents.
Cellular activity - Selectivity and Cytotoxicity Assay
| Bioassay | ML238 (IC50) |
|---|---|
|
Plasmodium falciparum Dd2 |
13 nM |
|
Mammalian cell cytotoxicity (Anti-Target) |
34,060 nM |
Summary /
ML238 showed an LC50 value of 34.06 μM in HEPG2 (Figure 1), while showing subnanomolar EC50 values in both 3D7 and Dd2 lines of P. falciparum (Table 1). This corresponds to a safety window greater than 30,000-fold. In addition, we have also screened the probe and several of its structural analogs for toxicity in kidney epithelial cells, dermal fibroblasts, lung epithelial cancer cells, and erythrocyte lysis (Table 2). The probe and selected analogs show excellent safety window.
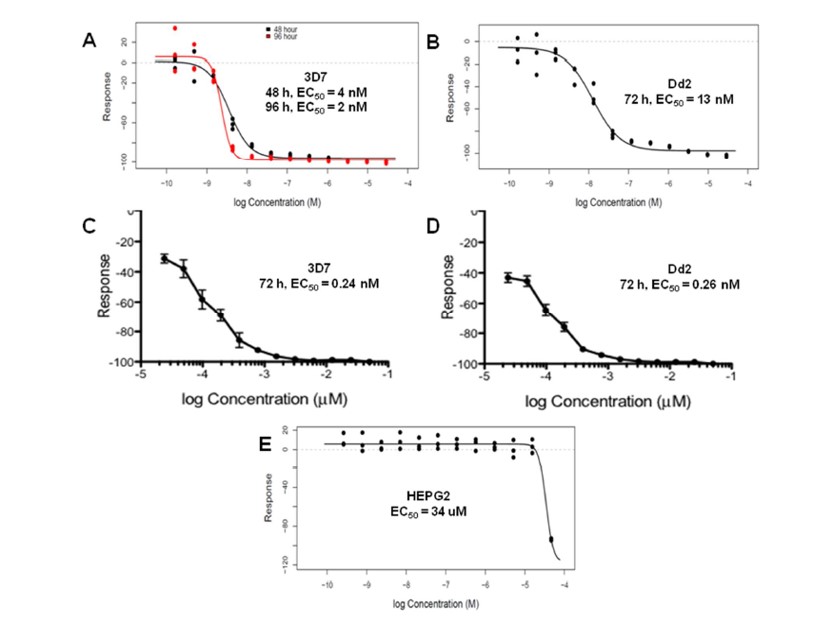
Figure 1. Dose-response curves for the probe (ML238) obtained using Luciferase assays against P. falciparum 3D7 (A) and Dd2 (B), and using a SYBR-green based assay in 3D7 (C) and Dd2 (D), low nanomolar to subnanomolar activity of the probe. The probe (ML238) only shows cytotoxicity in HEPG2 (E) at high micromolar, resulting in a large safety window.
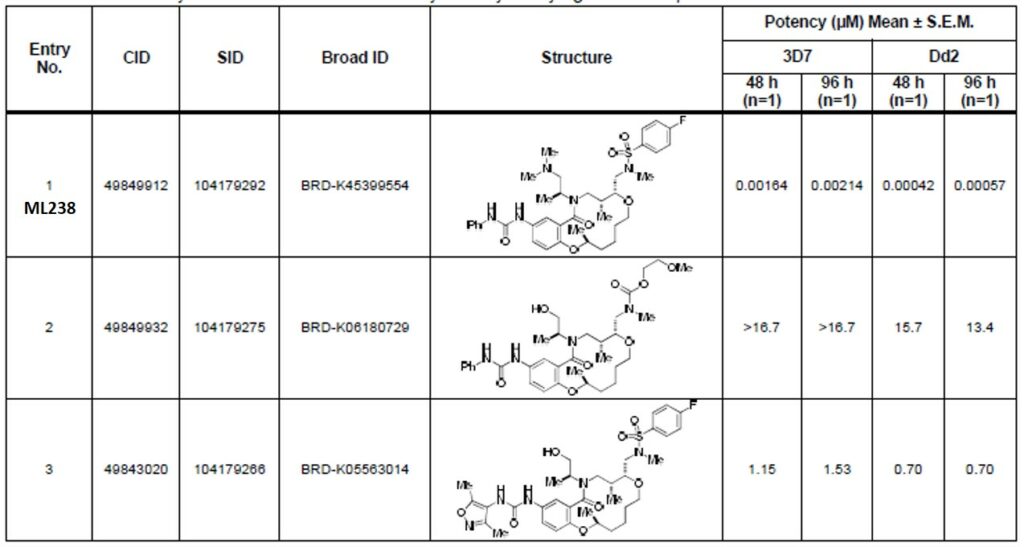
Table 1. Summary of Data Obtained in the Flow Cyclometry Assay Against P. falciparum after ML238 (and analogs) treatment.
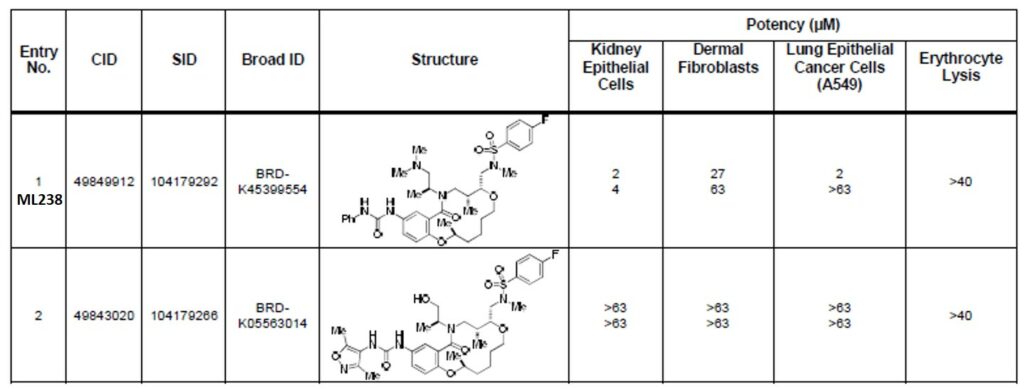
Table 2. Summary of Cytotoxicity Data in Kidney Epithelial Cells, Dermal Fibroblasts, Lung Epithelial Cancer Cells (A549) and Erythrocytes.
Cellular activity - Apicoplast Disruption Assay
Summary /
Delayed death inhibitors specifically target the parasite apicoplast; an organelle of cyanobacterial origin that employs prokaryotic-derived transcription and translation machinery (Lim 2010). During asexual bloodstage development, this organelle adopts an elongated and branched structure as the parasite matures into its trophozoite form, then segregates into individual organelles that parse with the individual daughter merozoites prior to their release from the infected erythrocyte. To verify if the compounds do interfere with the apicoplast, a whole-cell imaging screen with a recombinant parasite line that has GFP-labeled apicoplasts was used. This line, ACPL-GFP, has already been developed and benefits from chromosomal integration of the GFP fusion, producing excellent signal homogeneity. Antibiotics (such as zithromycin and doxycycline) that are known to interfere with the apicoplast have been observed to disrupt the morphology of the apicoplast in this GFP-expressing line (Figure 1) (Dahl 2007). Azithromycin was used as a positive control, while parasites treated with drug vehicle (medium containing 0.05 – 0.1% DMSO) served as a negative control. ML238 appeared to disrupt the apicoplast. The apicoplast morphology resembles the phenotype seen with chloroquine treatment; it is much smaller than in the untreated parasites but still present. Mitochondrial staining was generally diminished, though not entirely absent, in parasites treated with ML238 and some of its analogs. The mode of action of ML238 is still unknown. But its structure, very different from known antimalarial drugs and probes, suggests that its mode of action could involve a new target. In this context, target identification will help understanding the mechanism of action of the probe and will be a priority in the near future.
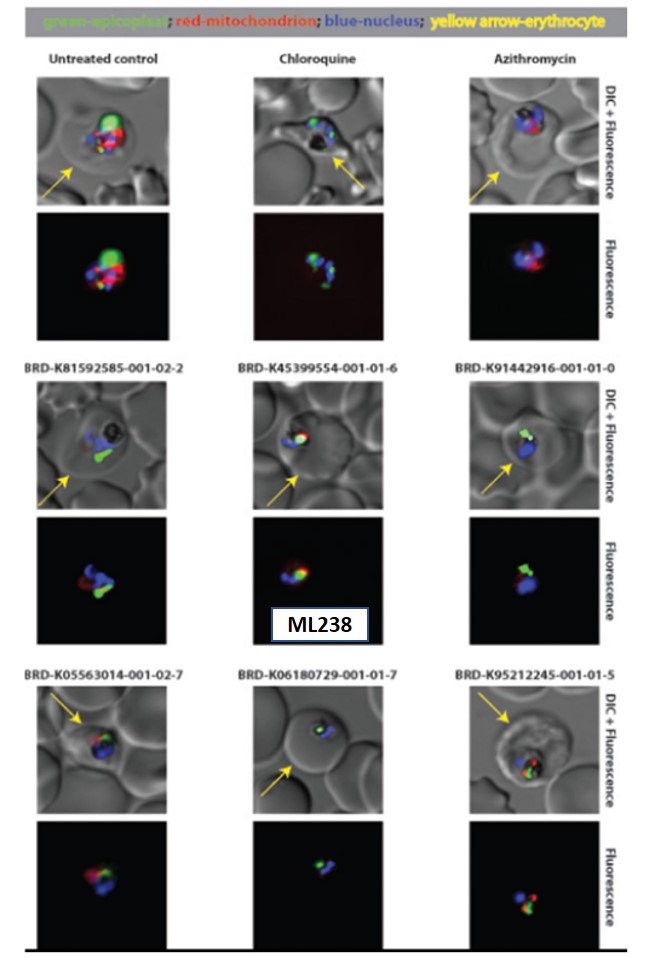
Figure 1. Parasites expressing GFP targeted to the apicoplast, 3D7 ACP(L)-GFP, were treated with the indicated compounds in 200 μL of culture media at a 1% hematocrit. At 96 hours, 30 μl of culture was harvested and stained with Hoechst (1 μg/ml) and MitoTracker Red (20 nM) for 30 minutes at 37 ºC. Stained parasites were imaged on a Nikon Ti inverted microscope with a 100X objective. The nuclei appear blue, mitochondria appear red, and the apicoplast appears green. The periphery of the infected erythrocyte is indicated with a yellow arrow. Drug concentrations: chloroquine, 16 nM; azithromycin, 80 nM; BRD-K81592585-001-02-2, 400 nM; ML238 (BRD-K45399554-001-01-6), 0.64 nM; BRDK91442916-001-01-0, 16 nM; BRD-K05563014-001-02-7, 400 nM; BRD-K06180729-001-01-7, 50 μM; BRD-K95212245-001-01-5, 0.64 nM.
References
- PubChem link: Quantitative high throughput screen for delayed death inhibitors of the malarial parasite plastid: Summary
- Weiwer M, Mulrooney C, Massi D, et al. ML238: An Antimalarial Small Molecule of a Unique Structural Class. In: Probe Reports from the NIH Molecular Libraries Program. Bethesda (MD): National Center for Biotechnology Information (US); April 14, 2011.
- Lim L, McFadden GI. The evolution, metabolism and functions of the apicoplast. Philos Trans R Soc Lond B Biol Sci. 2010;365(1541):749-763. doi:10.1098/rstb.2009.0273
- Dahl EL, Rosenthal PJ. Multiple antibiotics exert delayed effects against the Plasmodium falciparum apicoplast. Antimicrob Agents Chemother. 2007;51(10):3485-3490. doi:10.1128/AAC.00527-07


