ML303 : NS1 (Influenza Virus NS1A-Binding Protein) Antagonist
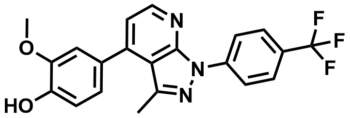
ML303
Target Name
Influenza Virus NS1A-Binding Protein
Target Alias
NS1
Target Class
Scaffold/Adaptor Protein
Mechanism of Action
Antagonist of NS1
Biological / Disease Relevance
Influenza NS1 Antiviral, Non Structural 1 Viral Protein
In vitro activity
NS1 Anti-Viral (A/PR/38) (IC90)Target Information
The Non Structural 1 (NS1) is a 230–237 amino acid relatively well-conserved viral protein that is usually expressed only during infection (Palese 2007). It contains an RNA binding domain (RBD) and an effector domain (ED) that is connected by a variable linker. The NS1 protein has been shown to be a key player that counters the host interferon (IFN) response to viral infection in strain-specific ways (Talon 2000). NS1 inhibits the function of OAS (2′–5′-oligoadenylate synthetase) and PKR (protein kinase R) (Silverman 2007) and block IFN-β synthesis at the post-transcriptional level by inhibiting the pre-mRNA splicing and blocking the export of poly(A)-RNAs from the nucleus to the cytoplasm (Qiu 1994). It also hampers the host’s defense pathways by interfering with the host RNAi pathway, adaptive immune response, and the apoptotic response (Nemeroff 1998). NS1 is also a key component in the temporal regulation of viral RNA synthesis, its splicing, and translation (Chen 1999). Thus, NS1 enhances the virulence of infection, posing as a compelling target for influenza treatment. These compounds will enable researchers to test how NS1 inhibition impacts the infection cycle and how antagonists can be leveraged alone or in combination with existing agents in the next generation of treatments for influenza infection. We developed and conducted a high-throughput screen using a novel yeast-based phenotypic assay to identify compounds which specifically inhibit NS1 function. Here we report a potent NS1 antagonist chemical series, represented by ML303. The SAR (structure activity relationship) around this chemical series was further developed by measuring the compound’s ability to inhibit replication of the influenza A/PR/8/34 virus in MDCK cells. The ML303 compound series specifically restored the NS1-inhibited expression of the interferon mRNA, inhibited the NS1 function in mechanistic assay and exhibited a potent antiviral activity in culture.
Properties

ML303
NCGC00248767
| Physical & chemical properties | ||||
|---|---|---|---|---|
| Molecular Weight | 399.4 g/mol | |||
| Molecular Formula | C21H16F3N3O2 | |||
| cLogP | 5 | |||
| PSA | 60.2 Ų | |||
| Storage | ||||
| Solubility | ||||
| CAS Number | 1638211-04-7 | |||
SMILES:
COC1=CC(C2=CC=NC3=C2C(C)=NN3C4=CC=C(C(F)(F)F)C=C4)=CC=C1O
InChI:
1S/C21H16F3N3O2/c1-12-19-16(13-3-8-17(28)18(11-13)29-2)9-10-25-20(19)27(26-12)15-6-4-14(5-7-15)21(22,23)24/h3-11,28H,1-2H3
InChIKey:
YBRHGNCGFMRGSV-UHFFFAOYSA-N
Activity
Summary activity statement /
ML303 (SID 124954998; CID 136141525) exemplifies a pyrazolopyridine chemical series, where several compounds show potent reduction of viral titer in MDCK cells infected with the Influenza A/PR/8/34 strain. This antiviral small molecule addresses the weak activity limitations of the prior art compounds as it showed improved potency of IC of 155nM without overt cytotoxicity to the host cells. ML303 can therefore be used by virologists as a tool to inhibit the function of the influenza NS1 viral protein to study its role in the antagonism of the host interferon system and its impacts on the viral infection cycle. Given the reasonable pharmacokinetics and ADME profile of ML303, this probe can also enabling research clinicians to test how it can be leveraged (alone or in combination) with existing agents in the next generation of treatments for influenza and other viral infections where the NS1 protein is crucial.
In vitro activity - Viral infection assay
Summary /
Triplicate cultures were infected with PR virus at MOI of 0.1% and treated with different concentrations of ML303. After 48 hour, the supernatants were collected and analyzed to determine the reduction in virus by assaying the supernatant infectivity. The data is presented as a percent decrease in titer (A) which is calculated from TCID where the probe IC is 155 nM and as the actual TCID (B) where 3 log units of viral suppression are observed at 1.6 μM concentration (AID 602450).
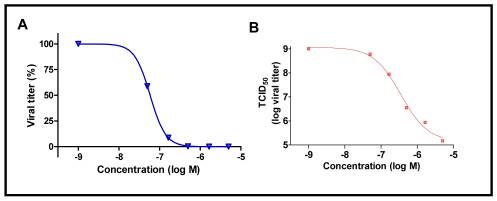
Figure 1. Dose response activity of ML303 on viral titer.
In vitro activity - ADME Profiling
Summary /
Preliminary ADME profiling revealed that ML303 and relative analogs have good stability in mouse live microsomes (MLL) (45% remaining after 30 minutes) and good permeability with low efflux in Caco-2 monolayer of cells (Table 1). ML303 and members of this series showed low solubility (< 50 μM), but this didn’t translate to poor in vivo pharmacokinetics as ML303 exhibited high exposure in plasma and lung after a single 30 mg/kg intra-peritoneal (IP) dose in mice and after 48 hours of probe treatment. It is well known that low solubility impacts permeability in Caco-2 assays, but the use of an appropriate formulation often results in reasonable in vivo bioavailability. Additionally the compound showed good stability in mouse plasma and PBS buffer.
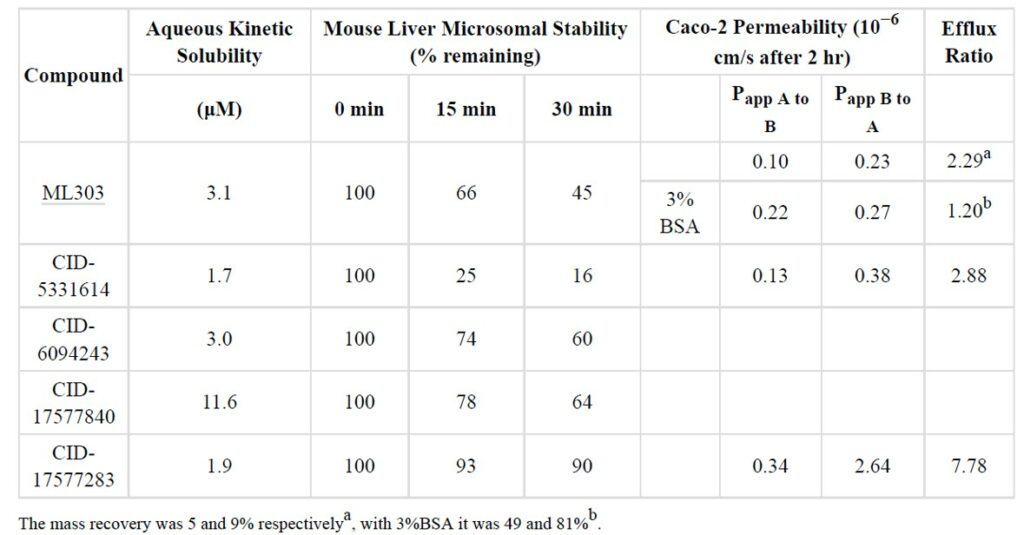
Table 1. In vitro ADME data for ML303 and relative active analogs.
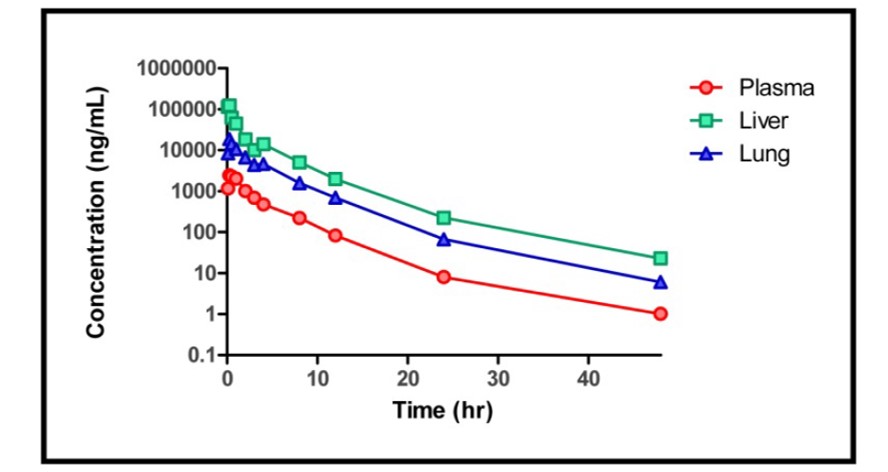
Figure 2. Time course PK plot of the ML303 concentration in plasma, liver and lung of IP treated mice. Each point represents the mean +/− SD of n=3.
Cellular activity - Yeast Growth Restoration Assay
Summary /
Growth of the control yeast strain pYES (without NS1) and the NS1 expressing stain NS1-PYES were measured as OD600 nm at 22, 42 and 60 hours. Results showed the slow growth phenotype exhibited by the NS1-pYes strain is reversed by the addition of 50 μM ML303 and after 60 hour the growth phenotype was comparable to that of the control treatments.
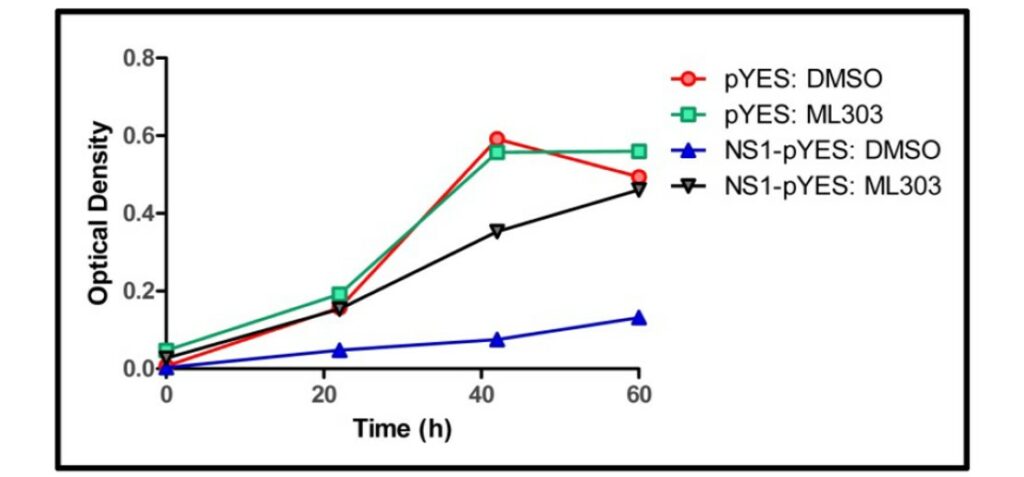
Figure 3. Compound-dependent restoration of yeast growth.
Cellular activity - Functional assay in MDCK cells
Summary /
Cells were mock infected (lane 2) or infected with influenza strain A/PR/8 at an MOI of 0.1 (lanes 4–9). Infected cells were treated with negative control 1% DMSO or different compounds (20 μM) including the probe ML303 (lane 7). After 6 hour, cells were harvested for RT-PCR analysis of IFN-β and β-actin mRNA levels. Results showed treatment with these compound restored IFN-β levels comparable to the positive control treatment poly (I:C) which directly induces IFN-β (lane 3) (AID 602456).
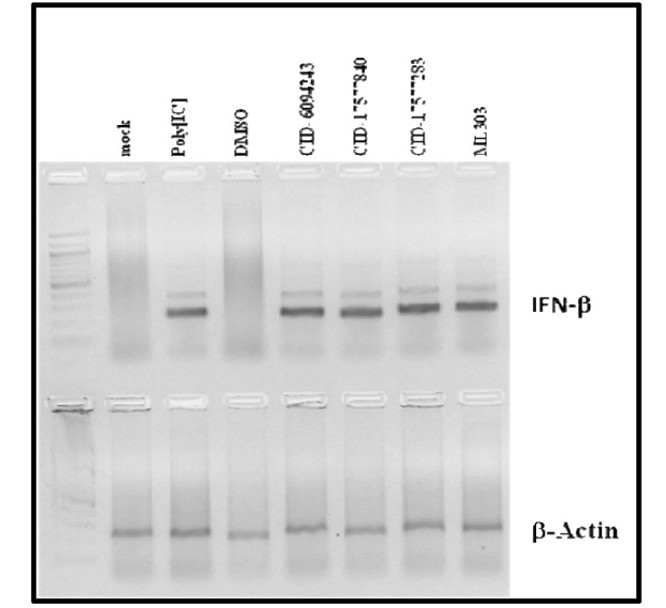
Figure 4. Compound-dependent restoration of IFN-β mRNA levels in MDCK cells.
References
- Summary Assay for Inhibitors of Influenza NS1 Protein Function
- Patnaik S, Basu D, Dehdashti S, et al. Discovery of Small Molecule Influenza Virus NS1 Antagonist. 2012 Apr 13 [Updated 2013 Sep 3]. In: Probe Reports from the NIH Molecular Libraries Program [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2010-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK169451/
- Palese P, Shaw ML. Fields Virology. 5 edition. 2007. Orthomyxoviridae: the viruses and their replication; pp. 1647–1689
- Talon J, Salvatore M, O'Neill RE, et al. Influenza A and B viruses expressing altered NS1 proteins: A vaccine approach. Proc Natl Acad Sci U S A. 2000;97(8):4309-4314. doi:10.1073/pnas.070525997
- Silverman RH. Viral encounters with 2',5'-oligoadenylate synthetase and RNase L during the interferon antiviral response. J Virol. 2007;81(23):12720-12729. doi:10.1128/JVI.01471-07
- Qiu Y, Krug RM. The influenza virus NS1 protein is a poly(A)-binding protein that inhibits nuclear export of mRNAs containing poly(A). J Virol. 1994;68(4):2425-2432. doi:10.1128/JVI.68.4.2425-2432.1994
- Nemeroff ME, Barabino SM, Li Y, Keller W, Krug RM. Influenza virus NS1 protein interacts with the cellular 30 kDa subunit of CPSF and inhibits 3'end formation of cellular pre-mRNAs. Mol Cell. 1998;1(7):991-1000. doi:10.1016/s1097-2765(00)80099-4
- Chen Z, Li Y, Krug RM. Influenza A virus NS1 protein targets poly(A)-binding protein II of the cellular 3'-end processing machinery. EMBO J. 1999;18(8):2273-2283. doi:10.1093/emboj/18.8.2273


