ML351 : ALOX15 (Arachidonate 15-Lipoxygenase) Inhibitor
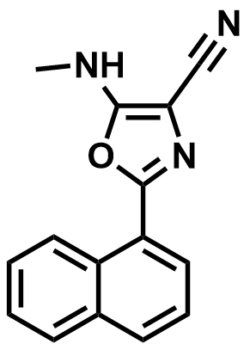
ML351
Target Name
Arachidonate 15-Lipoxygenase
Target Alias
ALOX15
Target Class
Oxygenase
Mechanism of Action
Inhibitor of ALOX15
Biological / Disease Relevance
15-LOX-1, Cancer Biology, Atherosclerosis, Neurodegenerative Conditions, Ischemic Stroke
In vitro activity
15-LOX-1 (IC50)Target Information
Lipoxygenases (e.g. 5, 12 and 15-LOX-1) are implicated in a number of human diseases, with reticulocyte 15-Lipoxygenase-1 (15-LOX-1 or 12/15 LOX) being specifically involved in cancer, atherosclerosis, and neurodegenerative conditions, such as stroke (Solomon 1997, Brash 1999, Berger 2007). Despite the potential therapeutic relevance of this target, few potent, selective and cell-active inhibitors have been reported. Toward this end, we employed a quantitative highthroughput (qHTS) screen against ∼74,000 small molecules which led to the discovery of ML351, a novel chemotype for 15 LOX-1 inhibition, that displays nanomolar potency (IC = 200 nM) and excellent selectivity (>250-fold) versus the related isozymes, 5-LOX, platelet 12-LOX, 15-LOX-2, bovine COX-1, and human COX-2. In addition, kinetic experiments were performed which indicate that this class of inhibitor is a tight binding, mixed inhibitor, which does not reduce the active-site ferric ion. Finally, ML351 protected against oxidative glutamate toxicity in mouse neuronal cells (HT-22) and significantly reduced infarct size in an in vivo mouse model for ischemic stroke. As such, ML351 represents the first report of a selective inhibitor of 15-LOX-1 with demonstrated in vivo activity in proof-of-concept models of stroke.
Properties

ML351
NCGC00070329
| Physical & chemical properties | ||||
|---|---|---|---|---|
| Molecular Weight | 249.27 g/mol | |||
| Molecular Formula | C15H11N3O | |||
| cLogP | 4.2 | |||
| PSA | 61.8 Ų | |||
| Storage | ||||
| Solubility | ||||
| CAS Number | 847163-28-4 | |||
SMILES:
CNC1=C(C#N)N=C(C2=CC=CC3=CC=CC=C23)O1
InChI:
1S/C15H11N3O/c1-17-15-13(9-16)18-14(19-15)12-8-4-6-10-5-2-3-7-11(10)12/h2-8,17H,1H3
InChIKey:
DYXYXTDIFMDJIR-UHFFFAOYSA-N
Activity
Summary activity statement /
ML351 (SID 104223766; CID 664510), exhibits potent and selective inhibition of 15-LOX-1 in vitro and demonstrates favorable cell permeability and activity in cell-based assays which enables researchers to study the role of 15-LOX-1 in a variety of biological systems. 15-LOX-1 has been implicated in the pathophysiology of stroke and neurodegenerative disorders (e.g. Alzheimer’s and Parkinson’s disease). As such, targeted inhibition of 15-LOX-1 has been proposed as a therapeutic strategy to mitigate the effects of these diseases by acting as neuroprotective agents. Importantly, ML351 has been shown to protect cells from oxidative stress-related neuronal cell death, efficiently cross the BBB, and significantly reduce infarct size in mouse models of stroke. These data suggest that ML351 can be used to probe the effects of 15-LOX-1 inhibition in animal models for a variety of neurodegenerative diseases.
In vitro activity - Selectivity and Cytotoxicity Assay
Summary /
Selective profiling of ML351 and selected analogs showed inactivity of ML351 against 15-LOX-2, 12-LOX and 5-LOX, but good inhibition against 15-LOX-1 with 0.02 μM (Table 1). Moreover, ML351 represents a vast PK and ADME property improvement over the majority of compounds reported previously (vide infra). Despite the low molecular weight (249 Da), and favorable log D (pH 7.4) of 2.6, most analogs exhibited poor solubility. The aqueous kinetic solubility in PBS buffer was determined to be 1.2 μM, which is about 7 times the in vitro IC50 . Empirically, a vast improvement in the solubility in the 15-LOX assay buffer was observed which was encouraging and suggests that solubility was not a detrimental factor in the biochemical studies. Importantly, the compound demonstrated favorable PAMPA permeability (passive) and acceptable Caco-2 permeability of >1 (1.5 cm/s^-6) with no evidence of efflux (efflux ratio: 0.7) suggesting the compound is not susceptible to the action of P-glycoprotein 1 (Pgp), a well-characterized ABC-transporter. Moreover, ML351 was stable in various aqueous solutions (pH 2, pH 7.4, pH 9) and mouse plasma. In addition, ML351 exhibited minimal CYP inhibition of the 2D6 and 3A4 isoforms at 10.3% and 3.5% inhibition respectively. Microsomal stability appears to be species dependent with ML351 possessing moderate stability to rat liver microsomes (T1/2 = 18 minutes) while being less stable to mouse liver microsomes (5.5 min). The compounds were completely stable in the absence of NADPH, suggesting a CYP-mediated degradation. Given the interest in testing compound ML351 in proof of concept mouse models of stroke, in vivo PK data on ML351 was obtained and found a suitable formulation. ML351 has a relatively fast half-life in both plasma and brain (T1/2 = ∼ 1 hr) with a Cmax of 13.8 μM in plasma (69 times in vitro IC50 ) and 28.8 μM in brain (144 times in vitro IC50 ). Encouragingly, ML351 has a brain/plasma ratio of 2.8 which demonstrates favorable BBB permeability and suggested that this compound was suitable for in vivo proof of concept (POC) models of ischemic stroke (vide infra).
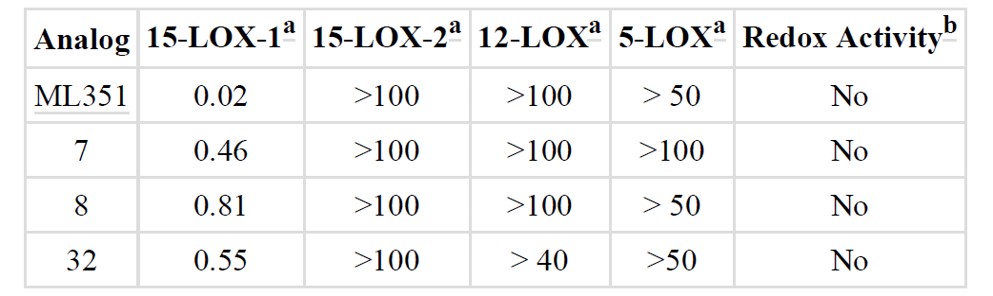
Table 1. Selectivity profiling of ML351 and other top compounds. (a) IC50 values are reported in micromolar. (b) UV-vis pseudoperoxidase activity assay was performed on all the four selected analogs and no degradation of the hydroperoxide product was observed at 234 nm, indicating a non-reductive inhibitory mechanism.
In vitro activity - Comparisson to Prior art
Summary /
Previously reported natural product based inhibitors of 15-LOX-1, such as boswellic acid, baicalein (Svensson 2013), and nordihydroguairetic acid (NDGA) (Whitman 2002) all possess several liabilities. These compounds are less potent and less selective towards 15-LOX-1, and are not easily amendable to further optimization as a result of their polyphenolic or terpenebased structures. In comparison, ML351 is potent (200 nM), selective (Table 2, no activity against related isozymes or COX-1/2)), chemically tractable and drug-like. Our previous chemical probe for 15-LOX-1, ML094, was found to be extremely potent (14 nM) and selective but lacked activity in cell-based assays, possibly due to poor cell permeability, intracellular hydrolysis of the terminal ester moiety, or inactivity against 12-LOX (Kenyon 2011). Tryptamine (37l, Figure 1) and imidazole-based (21n) inhibitors are potent and seemingly selective against 5-LO and 12-LO but a more comprehensive selectivity study is not reported. These compounds were reported to have unfavorable solubility and log P values, and while 21n seemed to possess improved physicochemical properties, with a ClogP of >5, there was no in vitro ADME or in vivo PK properties reported. Finally, the pyrazole derivative (15i) has improved solubility and cLogP, as compared to analogs 37n and 21n, suggesting inconclusive in vivo PK properties so despite the improved properties these compounds remain problematic. Additionally, the compounds reported by the BMS researchers, and in particular 21n and 15i, are quite complex structurally and possess at least one chiral center which limits their utility as probe compounds unless they are willing to provide academic researcher with the sample. In contrast, ML351 has much better ligand efficacy and can be synthesized in 2-3 steps from commercially available starting material. While ML351 does possess some aforementioned ADME liabilities (solubility, microsomal stability), this report represents the first account of a selective 15-LOX-1 inhibitor with demonstrated BBB permeability and activity in in vivo efficacy models for ischemic stroke.
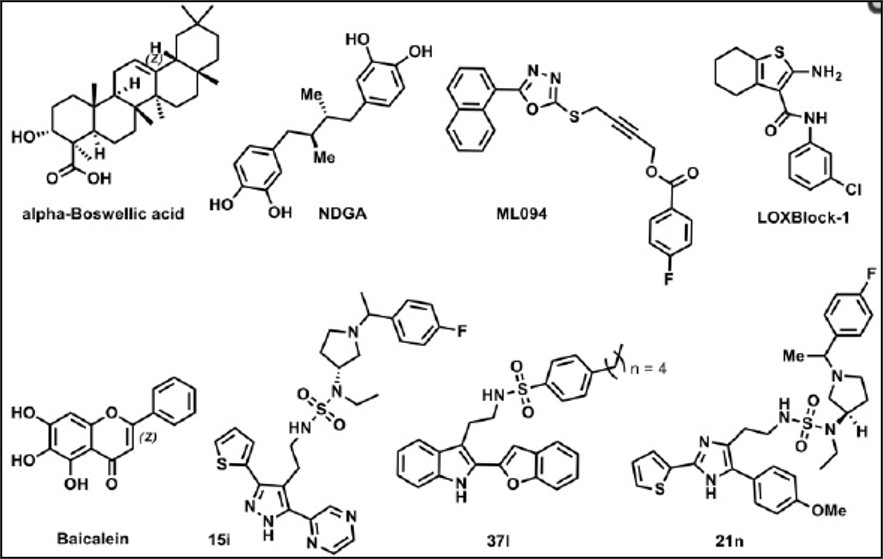
Figure 1. Prior Art Inhibitors of 15-Lipoxygenase-1 (15-LOX-1).
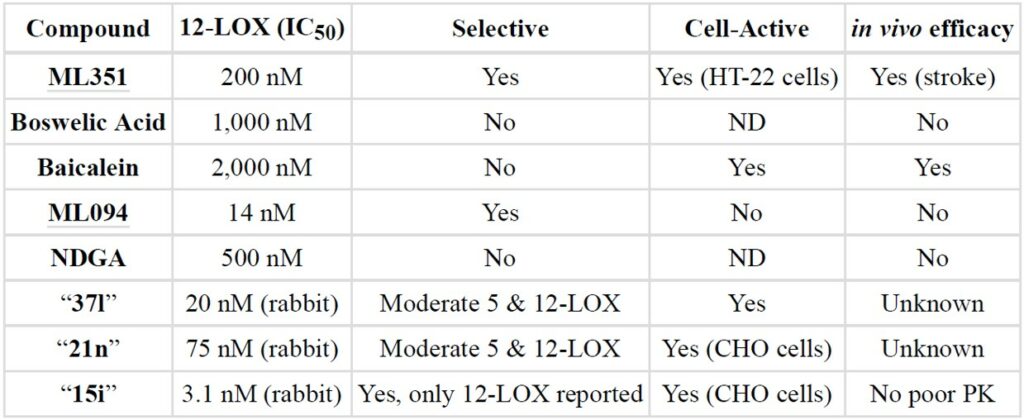
Table 2. Comparison of ML351 to previously identified 15-LOX-1 inhibitors.
Cellular activity - HT-22 cell (12-HETE Inhibition) functional assay
Summary /
HT-22 cells were treated with 5 mM glutamate alone or in the presence of ML351. Result showed inhibition of 12-HETE expression by the ML351 probe.
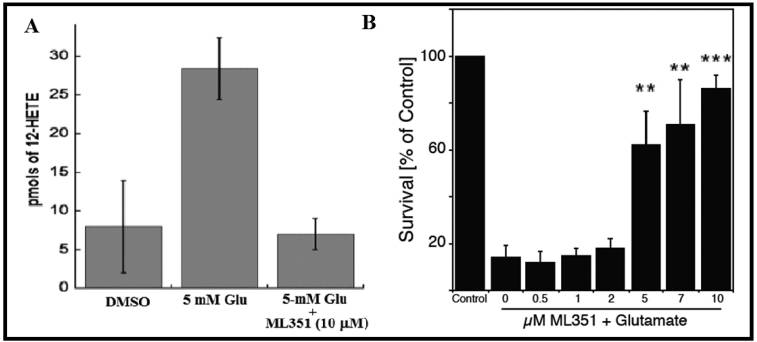
Figure 2. (A) Inhibition of 12-HETE production in HT-22 cells by ML351 following treatment with glutamate (5 mM). (B) Protection of Glutamate (5 mM) induced HT-22 death by increasing amounts of ML351 (** p < 0.1 *** P < 0.001). Result showed 12% death rate with no glutamate added (normalized to 100%).
In vitro and vivo activity - ADME Profiling and PK Studies
Summary /
ML351 represents a vast PK and ADME property improvement over the majority of compounds reported previously (vide infra). Despite the low molecular weight (249 Da), and favorable log D (pH 7.4) of 2.6, most analogs exhibited poor solubility. The aqueous kinetic solubility in PBS buffer was determined to be 1.2 μM, which is about 7 times the in vitro IC . Empirically, a vast improvement in the solubility in the 15-LOX assay buffer was observed which was encouraging and suggests that solubility was not a detrimental factor in the biochemical studies. Importantly, the compound demonstrated favorable PAMPA permeability (passive) and acceptable Caco-2 permeability of >1 (1.5 cm/s^-6 ) with no evidence of efflux (efflux ratio: 0.7) suggesting the compound is not susceptible to the action of P-glycoprotein 1 (Pgp), a well-characterized ABC-transporter. Moreover, ML351 was stable in various aqueous solutions (pH 2, pH 7.4, pH 9) and mouse plasma. In addition, ML351 exhibited minimal CYP inhibition of the 2D6 and 3A4 isoforms at 10.3% and 3.5% inhibition respectively. Microsomal stability appears to be species dependent with ML351 possessing moderate stability to rat liver microsomes (T1/2 = 18 minutes) while being less stable to mouse liver microsomes (5.5 min). The compounds were completely stable in the absence of NADPH, suggesting a CYP-mediated degradation. Given the interest in testing compound ML351 in proof of concept mouse models of stroke, in vivo PK data on ML351 was obtained and found a suitable formulation. ML351 has a relatively fast half-life in both plasma and brain (T1/2 = ∼ 1 hr) with a Cmax of 13.8 μM in plasma (69 times in vitro IC50 ) and 28.8 μM in brain (144 times in vitro IC50 ). Encouragingly, ML351 has a brain/plasma ratio of 2.8 which demonstrates favorable BBB permeability and suggested that this compound was suitable for in vivo proof of concept (POC) models of ischemic stroke (vide infra).
References
- Probe Development Summary of Inhibitors of 15-hLO-1 (15-human lipoxygenase 1)
- Rai G, Joshi N, Perry S, et al. Discovery of ML351, a Potent and Selective Inhibitor of Human 15-Lipoxygenase-1. 2013 Apr 15 [Updated 2014 Jan 13]. In: Probe Reports from the NIH Molecular Libraries Program [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2010
- Solomon EI, Zhou J, Neese F, Pavel EG. New insights from spectroscopy into the structure/function relationships of lipoxygenases. Chem Biol. 1997;4(11):795-808. doi:10.1016/s1074-5521(97)90113-7
- Brash AR. Lipoxygenases: occurrence, functions, catalysis, and acquisition of substrate. J Biol Chem. 1999;274(34):23679-23682. doi:10.1074/jbc.274.34.23679
- Berger W, De Chandt MT, Cairns CB. Zileuton: clinical implications of 5-Lipoxygenase inhibition in severe airway disease. Int J Clin Pract. 2007;61(4):663-676. doi:10.1111/j.1742-1241.2007.01320
- Whitman S, Gezginci M, Timmermann BN, Holman TR. Structure-activity relationship studies of nordihydroguaiaretic acid inhibitors toward soybean, 12-human, and 15-human lipoxygenase. J Med Chem. 2002;45(12):2659-2661. doi:10.1021/jm0201262
- Kenyon V, Rai G, Jadhav A, et al. Discovery of potent and selective inhibitors of human platelet-type 12- lipoxygenase. J Med Chem. 2011;54(15):5485-5497. doi:10.1021/jm2005089
- Svensson Holm AC, Grenegård M, Ollinger K, Lindström EG. Inhibition of 12-lipoxygenase reduces platelet activation and prevents their mitogenic function. Platelets. 2014;25(2):111-117. doi:10.3109/09537104.2013.783688


